Biological Interactions: Drug and Receptor Interaction, Agonis and Antagonism, Shape and Isomerism and the Role of Water
In Lecturio - Drug and receptor interaction is an important field of study in medicine. Knowing the chemistry behind these interactions leads to understanding how drugs work in the treatment of different ailments. In this article, the basic chemistry of receptors found in biological membranes is discussed. Also included in this article are the different types of interactions possible between the drug and receptors.
Table of Contents
Are you more of a visual learner? Check out our online video lectures and start your chemistry course now for free!
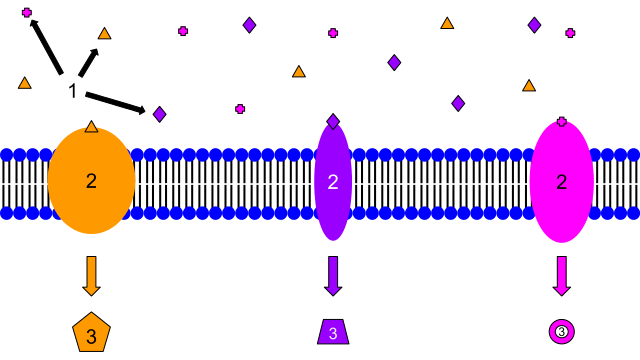
Image: “1. Ligands 2. Receptors 3. Secondary Messengers. These are examples of membrane receptors. Typically, they are proteins that are embedded in the membrane. Although there are many different ligands located outside of the cell, membrane proteins are specific, and only certain ligands will bind to each one. That is why each protein has a different ligand, and also induces a different cellular response. The response may be transcription of a gene, cell growth, or many other cellular actions.” by Isaac Webb. License: CC BY-SA 3.0
Receptors and Ligands
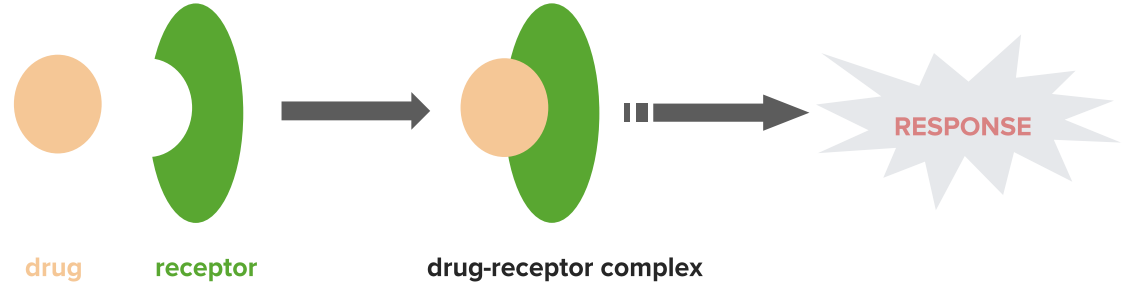
Note: A receptor is a site where a drug binds and then the receptor brings about a physical response.
Receptors are specific areas in proteins and glycoproteins embedded in the cellular membrane or in the nuclei of living cells. Ligands may selectively bind to these receptors. Binding of ligands is significantly affected by the stereoelectronic structure of compounds which will result in specific types of interactions with the receptors.
The ligands usually selectively bind with the receptors with complementary properties with it. When ligands attach themselves to these receptors, a biological response – positive or negative – will occur. Positive responses involve promoting or activating physiological responses, such as the opening of ion channels. Negative responses occur if the reverse happens. The binding of the ligand to the receptor causes inhibition of physiological responses.
- The drug is a ligand for this receptor
- It is described as an agonist
- In the case of the muscle relaxants, the receptor was identified as being in a family called the cholinergic receptors
- A naturally occurring agonist at this class of receptors is acetylcholine:
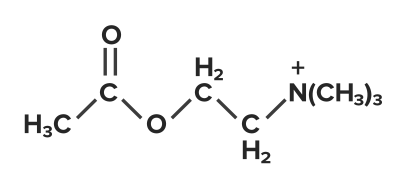
Acetylcholine
- Since the receptor is now blocked, acetylcholine cannot bind
- Since acetylcholine cannot act, the muscle contracts
- Drugs which block active sites without giving rise to a response are called antagonists
- Other active sites where drugs can bind include enzymes
This apparent contradiction was resolved by the introduction of the idea of a receptor. A receptor is a site where a drug binds and then the receptor brings about a physical response.
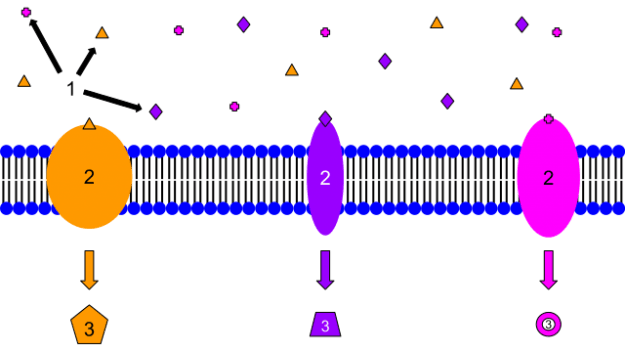
Image: “1. Ligands 2. Receptors 3. Secondary Messengers. These are examples of membrane receptors. Typically, they are proteins that are embedded in the membrane. Although there are many different ligands located outside of the cell, membrane proteins are specific, and only certain ligands will bind to each one. That is why each protein has a different ligand and also induces a different cellular response. The response may be a transcription of a gene, cell growth, or many other cellular actions.” by Isaac Webb. License: CC BY-SA 3.0
Response
Note: The drugs found to contract muscle were shown to be acting at the same receptors. These were found to bind to the receptors but without giving rise to a physical response.
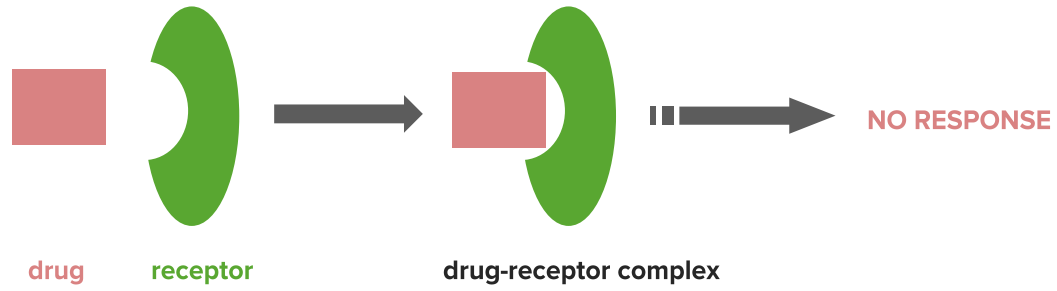
Agonism and Antagonism
Drugs are recognized by their targets by a number of types of interaction.
Drugs binding at the same site but in a different way can give rise to different effects (e.g. agonists and antagonists). Knowledge of these interactions allows us to work out how the drug binds and design new drugs and predict how they will bind.Types of Interaction
The binding of ligands to receptors are governed by the concept of chemical bonding. Intra and intermolecular forces of attraction play a big role in understanding the binding chemistry between the ligands and the receptors. These interactions include covalent bonding, ionic bonding, and dipole-dipole interactions. When the ligand approaches the receptor in an appropriate distance, a bond is formed, and then the drug action happens.This type of bonding is the strongest form of bond that can be formed between a ligand and a receptor. The bond formed is so very strong that detachment of the ligand is very hard and non-spontaneous. This type of interaction is usually not employed in drug action.
Ionic interactions are more reversible, compared to its covalent counterpart. This type of bond is effective at greater distances between the charges. The strength of this interaction is dependent on the distance between the charges.
| Bond type | Approx. energy/ kJ/ mol |
| covalent (single) | 300—450 |
| ionic | 20—40 |
| ion-dipole | up to 150 |
| hydrogen | 37 |
| dipole-dipole | 5 |
| hydrophobic | 4 |
| Van der Waals | 1 |
Electrostatic Interactions
In an electronic dipole, a polarized bond is formed. In it, there is a partially positive and partially negative end. The interaction between the positive and negative ends of different compounds form electrostatic bonds with other polar molecules or ionized compounds.Dipole-Dipole Interaction
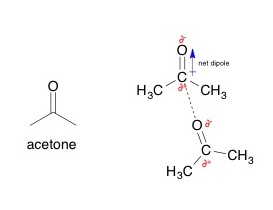
Image: “Dipole-dipole interactions between two acetone molecules, with the partially negative oxygen atom interacting with the partially positive carbon atom in the carbonyl.” by AviMole602. License: CC BY-SA 3.0
In many molecules, there are permanent dipoles due to the difference in electronegativities of atoms sharing chemical bonds.
Ion-Dipole Bonds
In ion-dipole interactions, an ion close to a polar molecule will be attracted to the end of the polar molecule which has a partial opposite charge with it. When they approach the appropriate distance between the two molecules, an electrostatic interaction will happen. This will mean a positive ion will go near the negative end of the polar molecule, while a negative ion will approach the polar molecule in its positive end.- Many drug molecules will have ionized functional groups
- The charges on these groups will bind with permanent dipoles, e.g.
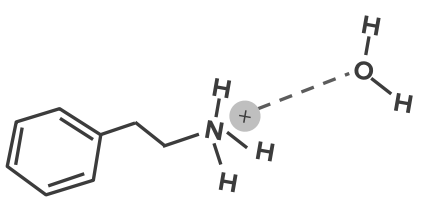
- This type of bonding play a key role in the water solubility of a drug
A salt of a weak base and a weak acid is not necessarily very water soluble.
Charge-Transfer Complexes
This type of complexes is formed between electron donor groups adjacent to electron acceptor groups. The relative positive or negative molecules transfer portions of its charges to the other molecule. In the process, a weak electrostatic bond is formed.Hydrophobic Bonding
This is a very weak form of bonding which only occurs when the non-polar sections of molecules aggregate together in the presence of water because of the low water solubility. Hydrophobic molecules don’t like to interact with polar water molecules, and so when water is present, their tendency is to aggregate and be closer to each other.Van der Waals Bonding
Van der Waal´s bonds exist between all atoms. They arise because the electron cloud associated with an atom or molecule is constantly moving so that the electrons are never evenly distributed. Small, local, instantaneous dipoles (charge separations) occur. Diploes behave like small magnets and will attract one another.Alkene Nomenclature
Van der Waal´s forces are weak. The larger the surface area and the larger the number of electrons in the molecules the larger the interaction will be. These interactions will only occur between molecules very close together: 0.4—0.6 nm apart. The forces drop off quickly if the molecules move apart: Force a 1/d6 where d = distance between.
These forces are insignificant for individual atoms but can be important in parts of molecules with lots of atoms, especially if the surfaces of the molecules are the right shapes to allow a close fit.
Hydrogen Bonds
Hydrogen bonds are special types of dipole interactions. They are formed when there is functional groups present which contains N, O or S and there is H linked to one of these atoms.
E.g. two molecules of water:- Due to the difference in electronegativity between O and H the O-H bond is polarized.
- Lone pairs of electrons on the oxygen bond with the hydrogen which has a partial positive charge
The H is effectively shared between the donor and acceptor.
Donors can form 1 H bond for each H, acceptors can form 1 H bond for each electron Ione pair. H bonds are directional, i.e.:
Hydrogen bonds are important not only in drug-target interactions but also in holding together the structure of proteins and DNA.
Ionic Bonding
Ionic bonds, formed between species with opposite charges, are strong and can act across long distances. Drugs are often ionized and the active sites in receptors contain charged groups (carboxylic acids and amines).Covalent Bonding
The majority of the bonds within drugs and their targets will be covalent. A small number of drugs can also make covalent bonds with their targets. Covalent bonds are strong and hence drugs forming them will usually be permanently bound to their target. Some anti-cancer drugs alkylate DNA in tumor cells. The alkylated DNA cannot function and hence the cell dies, e.g. mechlorethamine.Example of DNA interstrand linking
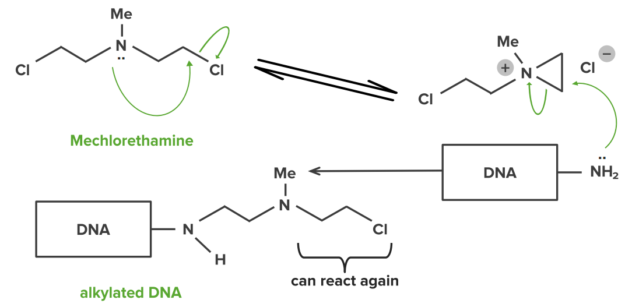
London Dispersion Forces
London dispersion forces may also cause interactions between a drug and a receptor. The interaction is very weak as there is no permanent dipole present in the molecules. This means the positive and negative ends only occur for a very short span of time. They usually arise because of the uneven distribution of electrons at a specific time period.Shape and Isomerism
In order for drugs to fit an active site, they must first have the correct shape.
Usually, only one isomer of a drug will have the required activity. Most natural molecules have stereocentres and occur in single optical isomers e.g. the amino acids which makeup proteins and hence receptors and enzymes.However, drugs should be administered as a single enantiomers as the mirror images may have other effects including:
- producing side effects
- countering the effect of the drug
- being metabolized to a toxic product
Geometrical isomerism
These occur when there is no free rotation about a bond, e.g. double bonds. The functional groups end up in different places.Conformational isomerism
Molecules can adopt preferred shapes although they can exist in other conformations, e.g. cyclohexane.
If the drug has a preferred conformation which fits the active site then it will usually bind more easily than if it needed to adopt an alternative shape think.
The Role of Water
Any system will adopt the lowest energy configuration. In chemical systems, this will mean that the participant will form as many bonds as possible of the strongest type. For example, water molecules will H-bond with each other; in ice, each molecule makes 4 H-bonds. In liquid water H-bonds are continuously forming and breaking—on average each molecule makes 3.4 H-bonds.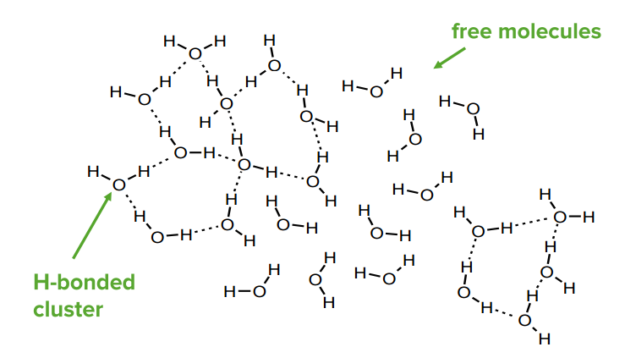
At any one time, there will be highly H-bonded clusters and areas with few H-bonds.
Review Questions
The correct answers can be found below the references.1. Which of the following interactions forms the strongest bond between the drug and the receptor?
- Ion-dipole
- Covalent bonding
- Ionic interaction
- Dipole-Dipole
- Acetone
- Water
- Ethanol
- Ethane
Comentários
Enviar um comentário