Calcium Homeostasis, Hypocalcemia and Hypercalcemia in Children — Diagnosis and Treatment
Calcium is an essential mineral. With a pivotal role in bone growth and neuromuscular development, it is of crucial importance in the pediatric population. This article envisages highlighting the significance of calcium homeostasis in children and gives an overview of the pathological states associated with abnormal calcium levels such as hypocalcemia and hypercalcemia.
Table of Contents
Are you more of a visual learner? Check out our online video lectures and start your pediatric endocrinology course now for free!

Definition of Calcium Homeostasis
Calcium is an essential mineral of critical importance. It is the most abundant mineral in the human body. Calcium affects a multitude of diverse bodily functions and is indispensable for life. Calcium level in the blood is controlled strictly by hormonal control and is also affected by a change in the blood level of pH and albumin. Calcium exists in the body in 2 forms, i.e., ionized and free. A tight control mechanism ensures optimum levels of ionized and total calcium in the body. Subtle changes in calcium levels can have stark repercussions. Calcium homeostasis is a crisply regulated essential function of the human body.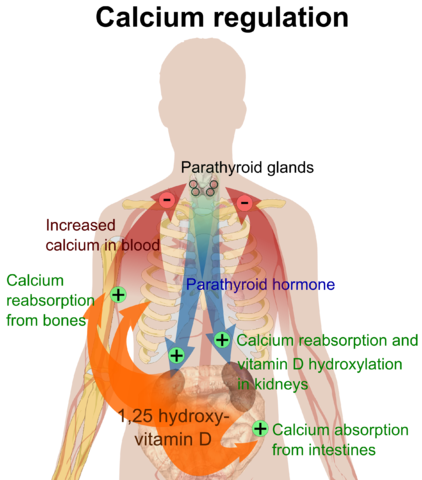
Image: “Overview of calcium regulation.” by Mikael Häggström. License: Public Domain
Key elements of calcium homeostasis
Maintenance of calcium level within a narrow range ensures optimum functioning of critical organs, such as the kidney and neurons. This regulation is multifactorial with a large number of checkpoints and quality control measures. The key elements of calcium homeostasis can be listed as follows:- Parathyroid hormone (PTH)
- PTH receptor
- Vitamin D
- Calcium-sensing receptor
Vitamin D
The discovery of vitamin D dates back to early 20th century when Elmer McCollum and Marguerite Davis were working meticulously to isolate the substance from cod liver oil that could serve as a cure for rickets in children.Today we know that vitamin D is a family of secosteroids which regulate the absorption of Calcium, Magnesium, and Phosphate and affect a multitude of biological processes in the human body.
The most important variant is vitamin D3, also known as cholecalciferol. The next most relevant secosteroid is ergocalciferol; also known as vitamin D2. Vitamin D2 and D3 are present in dietary supplements. The journey of vitamin D from its intake to activation and metabolism is complex. It reflects both spatial and temporal separation. The absorption of dietary vitamin D is a complex process involving both spatial and temporal separation.
Vitamin D2 and 3: Key steps in the process and their site of occurrence
Most of the Vitamin D is ingested; however, the majority of human vitamin D needs are satisfied through the synthesis of vitamin D from 7-dehydrocholesterol in the skin through UV-ray dependent chemical conversions.| Metabolic step | Explanation | Site of occurrence |
| Intake | Dietary intake: Vitamin D can be ingested in the form of vitamins D2 and D3. Main sources are plants, fish, fungi, and yeast. This state is, however, metabolically inactive. Vitamin D gets absorbed via chylomicrons and the lymphatic system. It then piggybacks its way through the circulation with vitamin D binding protein (DBP). Synthesis of skin: 7 dehydrocholesterol is converted first into pre-D3 and then vitamin D3 where the heat from the sun’s ultraviolet UVB radiation acts as a catalyst. | |
| Hydroxylation | The active form of vitamin D is created during this step. 25-hydroxycholecalciferol and 25-hydroxy ergocalciferol are the end products. These hydroxylated vitamin D metabolites are assessed in an individual’s serum to procure an estimate of the vitamin D level. | Liver |
| Hydroxylation | 25-hydroxycholecalciferol is further hydroxylated to form 1,25 hydroxycholecalciferol. The latter is the biologically active form of vitamin D and is also known as calcitriol. It is calcitriol which acts on the nuclear receptors in various cells and rolls in many biochemical cascades. By regulating this hydroxylation reaction; PI, Ca+2, FGF23 and other such factors maintain the level of the active vitamin. PTH also has regulatory power at this juncture. | Kidney |
| Excretion | The 24-hydroxylase enzyme is responsible for the breakdown of calcitriol into calcitroic acid. | Calcitronic acid is excreted in the urine. PTH inhibits excretion of calcitroic acid in urine. |
| Action | Calcitriol acts on nuclear receptors in different cells to activate varied chemical cascades. | Osteoblast: Both calcitriol and PTH act on osteoblast which, through RANKL and RANK interaction, activates preosteoclast to form osteoclast. The osteoclast acts on the bones to bring about calcium and phosphorus release. Intestine: Calcium and phosphate absorption is brought about by calcitriol. |
Relation of calcium to other constituents in blood
Calcium exists in the body in three forms, viz. ionized, bound to albumin and bound to anions. A subset (45 %) of the total circulating calcium is in ionized form. This ionized active calcium is responsible for its physiochemical effects and chemical interactions. The level of ionized calcium is, in turn, dependent on the other constituents of the serum. Consequently, an estimate of ‘true’ ionized calcium level needs to be considered in relation to these key regulatory elements. Further rectifications are done accordingly.A short accurate description of the interaction of calcium with the other key constituents of human serum is tabulated below:
| Constituents of Serum | Interaction |
| Phosphate | has negative regulatory authority on calcium. Increase in phosphate culminates in decrement in serum calcium levels. |
| Magnesium | Fall in serum magnesium level induces hypocalcemia. |
| Albumin | Some circulating calcium is bound to albumin. One gram reduction in serum albumin levels demands 0.8 mg/dL increase in the serum calcium level. Total calcium needs to be thus corrected if there is low albumin. |
Functions of Calcium
Calcium has broad effects throughout the body, and that range must be very tightly maintained to keep action potentials working across nerve cells. The most significant functions of calcium are enlisted as follows:- Bone mineralization
- Metabolic functions
- Neuromuscular functions
- Regulation of clotting mechanisms
- Effective intracellular messenger for variety of substances such as Insulin
| Modality | Level |
| Plasma total calcium level | 9–10.5 mg/dL |
| Serum ionized calcium concentration | 4.5–5.6 mg/dL |
Pathology of Calcium Homeostasis: Hypocalcemia
Definition
The state of low serum calcium is termed as ‘Hypocalcemia’. Mild disease is asymptomatic. Severe hypocalcemia can be potentially life-threatening and can present in any of the following manners:- Seizure
- Tetany
- Diffuse spasm
- Arrhythmia
- Chvostek’s and Trousseau’s sign
Diagnosis
Laboratory TestsFor a patient with unexplained hypocalcemia, following labs are useful:
- 1,25 OH vitamin D
- 25 OH vitamin D
- Serum intact PTH
- Basic metabolic panel
- Magnesium and phosphate
- Alkaline phosphate
- Urine Ca:Cr ratio
Causes
Based on the above-mentioned tests, the most common and relevant causes of hypocalcemia can be summarized as shown below:| Diagnosis | Phosphate level | PTH measure | 25-OH Vitamin D assessment | 1,25-OH Vitamin D measure | Other labs |
| Vitamin D deficiency | ↓ | ↑ | ↓ | ↔ | Increase in alkaline phosphatase levels with decreased urine calcium measure is seen. |
| Hypoparathyroidism | ↑ | ↓ | ↔ | ↓ | Increase in urine calcium levels is documented |
| McCune Albright’s disease | ↑ | ↑↑ | ↔ | ↓ | Urine calcium increases |
| Renal failure | ↑ | ↑ | ↔ | ↓ | Increased in creatinine, a hallmark of renal failure is documented. |
There are few salient points one should be wary of while dealing with hypocalcemia. The same has been tabulated for easy understanding.
- Check an EKG to see if there is prolonged QRS interval (risk for arrhythmia)
- For hypoparathyroidism, consider DiGeorge syndrome
- Check exam and X-ray of chest and extremities for signs of rickets
Management
Hypocalcemia, when mild, can be managed on oral calcium supplementation alone. However, severe hypocalcemia calls for emergent dedicated multi-pronged treatment to avoid serious morbidity and even mortality.The most relevant aspects of management of hypocalcemia have been summarized below:
- 10 % calcium gluconate bolus for severe hypocalcemia
- Supplement calcium and vitamin D as needed
- Calcitriol (1,25 OH vitamin D) for renal failure, hypoparathyroidism, McCune Albright’s syndrome
Pathology of Calcium Homeostasis: Hypercalcemia
Definition
Hypercalcemia epitomizes the congregation of signs and symptoms when calcium levels escalate beyond the physiological upper limit. The saying “Stones, Bones, Groans and Psychiatric Overtones” engulfs the major features of hypercalcemia in a poetic but poignant manner.Severe hypercalcemia is characterized by calcium levels more than 14 mg/dL. With calcium levels so high, there is a potential risk of complete neuro-musculoskeletal breakdown and cardiac and renal system collapse. Severe hypercalcemia, hence, is dreaded and calls upon rapidly integrated management.
Etiology
Various distinct etiologies are associated with hypercalcemia.In infants, the most relevant pathologies associated with hypercalcemia can be summarized as follows:
- Maternal hypocalcemia
- Williams syndrome
- Familial hypocalciuric hypercalcemia
- Fat necrosis
Signs and Symptoms
The signs and symptoms of hypercalcemia in various pediatric age group patients can be summarized as follows:- Vomiting
- Dehydration from polyuria
- Dysmorphic symptoms associated with William syndrome
Causes
The circumstances in which older children develop hypercalcemia are quite different. The major causes of hypercalcemia in older children can be tabulated as follows:- Sarcoidosis, tuberculosis and other causes of granulomas
- Malignancy
- Hyperparathyroidism
- Vitamins and medications:
- Increased vitamin A and D
- Thiazides
- Lithium
Diagnosis
Diagnosis of hypercalcemia can be established based on simple blood tests alone. Assessment of serum calcium and serum PTH level suffices to establish the diagnosis and confirm the most common etiologies. Once, escalated calcium levels are documented, basic discrimination among the various etiologies starts with determination of PTH level. A small and simple algorithm is as follows:| PTH level | Correlation |
| Increased PTH | Hyperparathyroidism |
| Decreased PTH | One should commence the next step of determining the PTHrP |
| Normal PTH | Normal PTH is seen in familial hypocalciuric hypercalcemia (FHH) |
Management
As mentioned earlier, severe hypercalcemia is a threat to the critical cellular pathways essential for survival. The key features in the management of hypercalcemia can be summarized as follows:- The most important primary step is to bring about volume expansion and positively evade dehydration.
- Calcitonin may be thought of as an antidote. However, one should be cautious of overenthusiastic repeated use of calcitonin, as the same is associated with tachyphylaxis.
- Bisphosphonates are used.
- Dialysis is considered in cases of severe renal infliction.
- Steroids may be effective for the granulomatous disease.
- Parathyroidectomy for hyperparathyroidism.
Summary
Calcium is an essential mineral element and one of the most crucial building blocks of life.Calcium homeostasis is largely determined by vitamin D, PTH, PTH receptor and calcium-sensing receptor. A strict regulation of calcium level within a relatively narrow range is a prerequisite for optimum functioning of the neuromuscular system.
Vitamin D is procured by the body through diet and dietary supplementation as cholecalciferol and ergocalciferol. The human skin possesses a unique set of chemicals to produce vitamin D using specifically UVB radiation from sunlight.This vitamin D configuration is biologically inactive. Further hydroxylation in the liver and then kidney results in the synthesis of the active form of vitamin D, also known as calcitriol. PTH regulates vitamin D synthesis and activation.
Ionized calcium is responsible for the physiochemical effects of calcium. Ionized calcium levels are altered as a result of interaction with other constituents of the blood serum, such as phosphate and magnesium. For every 1 gm/dL fall in albumin, 0.8 mg/dL of calcium needs to be added to the total calcium estimate to obtain the true calcium level.
Calcium is indispensable for survival. It serves a multitude of functions, such as regulation of bone mineralization, clotting mechanism activation, and regulation of various processes and steps in the neuromuscular system.
Total calcium level is about 9–10.5 mg/dL. Serum ionized calcium assessment reveals that about half of serum calcium exists in ionized form.
Pathological aberrations in the level of calcium in the blood are dangerous. While mild abnormalities are relatively well tolerated, severe fluctuations can inflict severe harm on the cardiac, neuromuscular, and skeletal system. Both hypocalcemia and hypercalcemia come with a unique set of symptoms and signs. Quick recognition of these circumstances and dedicated management can help evade long-term damage
Comentários
Enviar um comentário