Pharmacokinetics and Pharmacodynamics
For drugs to produce significant effects, they must be able to interact with the body. This interaction is affected by a number of factors which include the properties of the drugs and mechanism by which drugs are absorbed through the body systems. In this article, basics of pharmacokinetics and pharmacodynamics are discussed. Given emphasis are some of the factors affecting drug action, namely lipophilicity, acid sensitivity and BBB penetration.

Drugs usually work by binding to a receptor and producing the desired effects. The concentration of drug present at the site of the receptor determines the intensity of the effect of the drug.
Other factors affecting the drug responses include the mechanism by which signal is transmitted into the cell by secondary messengers, some receptors present on the cell surface, and other regulatory factors that control gene translation and protein synthesis.
For a drug to be considered effective, the drug must be readily absorbable in sufficient quantity. If the drug cannot be absorbed readily by tissues, no drug effect will happen. The drug should also be easy to distribute to the target tissues. Drugs should be able to attach themselves to target tissues to lessen its unnecessary effects to other tissues. The last important characteristic of a good drug is that it is not metabolized very quickly. This is an important factor so that it can maximize its effect to the target tissues.
In some instances also, the inclusion of polar groups in the drug structure may be important. This is mostly the case when the drug is too lipophilic that may inhibit drug-receptor interactions.
Because of this, there is a need to incorporate an electron-withdrawing group to reduce nucleophilicity of the compound, making it less susceptible to acid hydrolysis. Incorporating a benzene ring in the sides of the penicillin can make the penicillin compound less sensitive to acids, increasing the possibility of drug action.
It was later then discovered that the central nervous compartments contain layers of tightly packed endothelial cells that can only allow small lipophilic drugs to cross by diffusion. The barrier consists of at least one lipid membrane that can accommodate hydrophilic molecules to enter into the extracellular spaces of the brain.
The drug molecules should be able to penetrate the blood-brain barrier for drug action to occur.
Example: Morphine, a very strong analgesic, has only small penetrating ability into the blood brain barrier because of the presence of the two OH groups in its structure. Masking these OH groups with non-polar groups increases the lipophilicity of the drug, thereby increasing its penetrating ability.
Compared to morphine, the structure of codeine includes a methyl group attached to the hydroxyl oxygen. Since the addition of an alkyl group decreased the polarity of the original OH group in morphine, codeine has ten times more penetrating ability compared to morphine. Higher is the effect of esterifying the –OH groups of morphine with acetate groups in a diamorphine molecule. Since both OH groups are masked in the process, the molecule becomes more lipophilic and eventually increased BBB penetration by 100 times.
1. What do you call the study that deals with the time course of drug absorption, distribution, metabolism, and excretion?
Table of Contents
Are you more of a visual learner? Check out our online video lectures and start your pharmacology course now for free!

Pharmacokinetics
It is defined as the study of the kinetics of drug absorption, distribution, excretion, and metabolism. Drug scientists study the pharmacokinetics of drugs to enhance the efficiency of drug delivery and, in the process, reduce the risk of toxicity to the patient during drug therapy. Different drugs tend to have different drug kinetics. There are also some drug delivery systems available that can enable drug scientists to study different rates of drug release.Pharmacodynamics
There is a direct correlation between the drug concentration and its effect on patients. This relationship is studied by describing pharmacodynamics of drugs. Pharmacodynamics specifically refers to the relationship of drug concentration at the site of action and the resulting effects. Also included in the study of pharmacodynamics are the time course and intensity of the therapeutic and adverse effects.Drugs usually work by binding to a receptor and producing the desired effects. The concentration of drug present at the site of the receptor determines the intensity of the effect of the drug.
Other factors affecting the drug responses include the mechanism by which signal is transmitted into the cell by secondary messengers, some receptors present on the cell surface, and other regulatory factors that control gene translation and protein synthesis.
Drug Action
Drug action usually occurs in three phases:- Pharmaceutical phase
- Pharmacokinetic phase
- Pharmacodynamic phase
For a drug to be considered effective, the drug must be readily absorbable in sufficient quantity. If the drug cannot be absorbed readily by tissues, no drug effect will happen. The drug should also be easy to distribute to the target tissues. Drugs should be able to attach themselves to target tissues to lessen its unnecessary effects to other tissues. The last important characteristic of a good drug is that it is not metabolized very quickly. This is an important factor so that it can maximize its effect to the target tissues.
Overview: principle phases in drug action
| Classification | Pharmaceutical Phase | Pharmacokinetic Phase | Pharmacodynamic Phase |
| Process taking place | Disintegration of dosage form Dissolution of active substances | Absorption Distribution Metabolism Excretion | Drug-receptor (or enzyme) interaction in target tissue |
| Objective | Optimisation of pharmaceutical availability (drug available for absorption) | Optimisation of biological availability (drug available for action) | Optimisation of required biological effect (induction of therapeutic effect) |
Factors Affecting Drug Absorption
The rate of absorption of the drug is affected by some factors. These factors include lipophilicity, solubility, ionization, degradation, metabolism and physiology. Three factors affecting the drug absorption are given focus, namely lipophilicity, acid sensitivity, and BBB (blood-brain barrier) penetration.Lipophilicity
In general, the higher the lipophilicity of a drug, the higher its membrane permeability and the higher its metabolic clearance (first-pass effect).
Therefore, it is important to balance lipophilicity with metabolic susceptibility.
Drugs are sometimes modified to alter the properties of drugs. To make the drugs more lipophilic, the polar groups in the drug molecules should be masked. For example, the alcohol (-OH) groups of phenolic and alcoholic drugs can be converted to less polar esters or ether groups. These specific alterations are sometimes unwanted as these polar groups may have special interactions also with the receptors. In cases like this, prodrugs that can temporary mask the polar groups may be employed.Therefore, it is important to balance lipophilicity with metabolic susceptibility.
In some instances also, the inclusion of polar groups in the drug structure may be important. This is mostly the case when the drug is too lipophilic that may inhibit drug-receptor interactions.
Acid Sensitivity
Some drugs undergo structural transformations in the presence of acids. These structural transformations greatly affect the therapeutic actions of drugs. For example, penicillin undergoes internal transformations forming esters. These esters are highly sensitive to the presence of acid in the stomach undergoing in the process hydrolysis reaction. Because of this, even before absorption in the gastrointestinal tract, the esters are already hydrolyzed affecting the drug action.Because of this, there is a need to incorporate an electron-withdrawing group to reduce nucleophilicity of the compound, making it less susceptible to acid hydrolysis. Incorporating a benzene ring in the sides of the penicillin can make the penicillin compound less sensitive to acids, increasing the possibility of drug action.
BBB Penetration
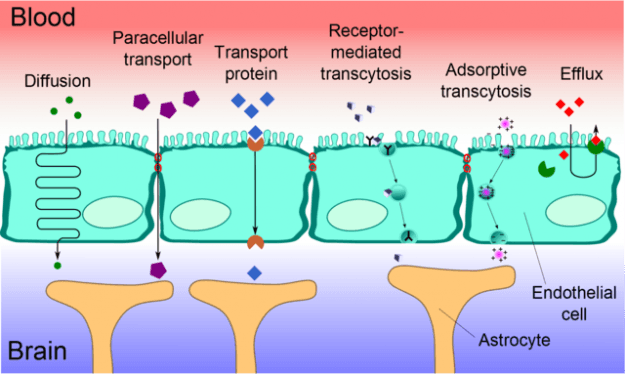
Membrane Barriers: Blood-Brain Barrier (BBB) – layer of tightly packed endothelial cells that allow only small (< 500 Da) lipophilic drugs to cross by diffusion.
In the late 1900s, Paul Ehrlich demonstrated the existence of Blood-Brain Barrier by injecting aniline dyes into the blood of experimental animals. He was able to observe that all of the organs of the animals were stained by the dye, except the brain.It was later then discovered that the central nervous compartments contain layers of tightly packed endothelial cells that can only allow small lipophilic drugs to cross by diffusion. The barrier consists of at least one lipid membrane that can accommodate hydrophilic molecules to enter into the extracellular spaces of the brain.
The drug molecules should be able to penetrate the blood-brain barrier for drug action to occur.
Example: Morphine, a very strong analgesic, has only small penetrating ability into the blood brain barrier because of the presence of the two OH groups in its structure. Masking these OH groups with non-polar groups increases the lipophilicity of the drug, thereby increasing its penetrating ability.
Compared to morphine, the structure of codeine includes a methyl group attached to the hydroxyl oxygen. Since the addition of an alkyl group decreased the polarity of the original OH group in morphine, codeine has ten times more penetrating ability compared to morphine. Higher is the effect of esterifying the –OH groups of morphine with acetate groups in a diamorphine molecule. Since both OH groups are masked in the process, the molecule becomes more lipophilic and eventually increased BBB penetration by 100 times.
Review Question
The answer can be found below the references.1. What do you call the study that deals with the time course of drug absorption, distribution, metabolism, and excretion?
- Pharmacokinetics
- Pharmacodynamics
- Drug Concentration
- Kinetic Homogeneity
Comentários
Enviar um comentário