Protein Movement and Cell Signaling
Proteins are important biomolecules because they serve a number of functions essential to metabolism. Discussed in this article are the different functions of proteins in the biological system. Specifically, the article discusses proteins and their structural functions. Another function given emphasis is the use of proteins in communication and signaling. Proteins and their role in movement and mobility are also discussed in this article.
Table of Contents
Are you more of a visual learner? Check out our online video lectures and start your biochemistry course now for free!

Example of signaling between bacteria. Salmonella enteritidis uses N-Acyl homoserine lactone for Quorum sensing
Protein Structural Consideration
With the variety of amino acids that can be incorporated in the structure of a protein molecule, protein can exhibit different structural properties. The difference in the structural properties enables proteins to serve different structural functions.Types of proteins found in Human Body
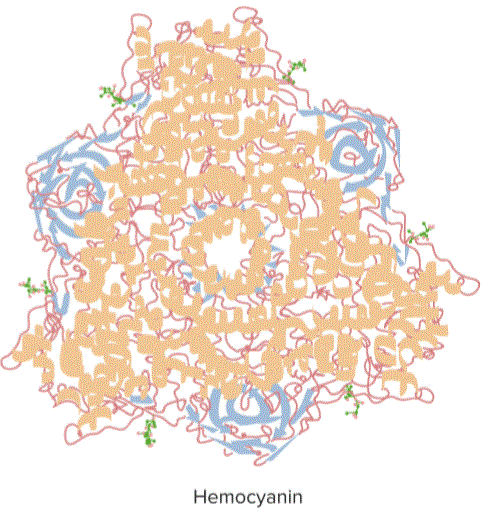
“Protein Structural Consideration” Image created by Lecturio
- Keratins in hair and nails
- Collagen in cartilage and connective tissues
- Fibroin in silk
- Elastin and fibrillin in connective tissues
- Lamin in the nuclear envelope
- Actin in cytoskeleton filaments
- Tubulin in microtubules.
Structure of Proteins
The primary structure of a protein involves the formation of peptide bonds between amino acids. The amino acid sequences for a specific protein are encoded in DNA. The side chains of amino acids enable different types of interaction within the molecule. These interactions cause the protein molecule to produce different secondary and tertiary structures. The chemical and biological properties of proteins depend more on their three-dimensional or tertiary structure.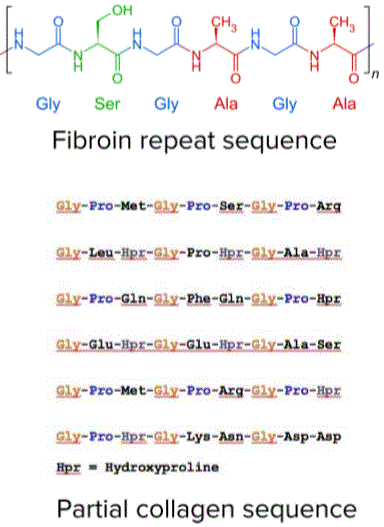
: “Fibrous Protein. Repeated Sequences” Image created by Lecturio
The tertiary structure of protein determines its over-all three-dimensional shape. A protein molecule tends to bend and twist so as to achieve maximum stability. The stabilizing forces are the bonding interactions between amino acid side chains.
Due to the solvent polarity around the protein molecules, hydrophobic amino acids tend to be buried in the interior of the protein molecule. Acidic and basic amino acids are the ones generally exposed because of their high hydrophilicity.
Structural Proteins
Proteins that serve different structural functions are divided into two types: fibrous and globular. Fibrous proteins are proteins that have thread-like structure. They may have normal helical or sheet structure; they exhibit comparatively stronger intermolecular forces of attraction. They are also relatively insoluble in water, acids, and bases. Globular proteins, on the other hand, have folded ball-like structure. Weak intermolecular hydrogen bonding governs the structure of this type of proteins. They are also soluble in water, acids, and bases.| Tubulin | Actin |
|
|
Fibrous proteins
Fibrous proteins mostly exhibit just secondary structures. Repeat sequences are also common in the structure of fibrous proteins. Most of the chains of amino acids may be linked by disulfide bridges. This disulfide linkage makes the protein very strong and stable. The most common fibrous proteins are collagen and keratin. Collagen is found in bone, cartilage, tendons and ligaments. This fibrous protein is strong, but is still very flexible. The repeat sequence for collagen is glycine-proline-X (any other amino acid). The combination of amino acids enables the collagen to be wound in tightly-coiled, straight or unbranched helices.Globular proteins
Globular protein changes conformation in such a way that the hydrophobic groups are inside the core of the protein, while the hydrophilic groups are exposed. This arrangement makes globular protein very soluble. The most common globular protein is hemoglobin. Hemoglobin is used to transport oxygen around the body. Its quaternary structure is made up of 4 polypeptide chains; two of which are identical alpha chains with 141 amino acids.The other two are identical beta chains with 146 amino acids. The four chains are linked to form a roughly spherical molecule. Attached to each of the polypeptide chains is a Fe2+ ion which can combine with one O2 molecule. Since there are 4 polypeptide chains, a maximum of four O2 molecules can be transported by a hemoglobin molecule.
Communication and Signaling
Cell signaling is a part of a complex communication system that regulates cell activity and coordinates cell actions. How cells respond to the microenvironment is the basis for its development, for tissue repair, and for immune response. Two of the primary functions of proteins in communication and signaling are membrane receptors, and signals.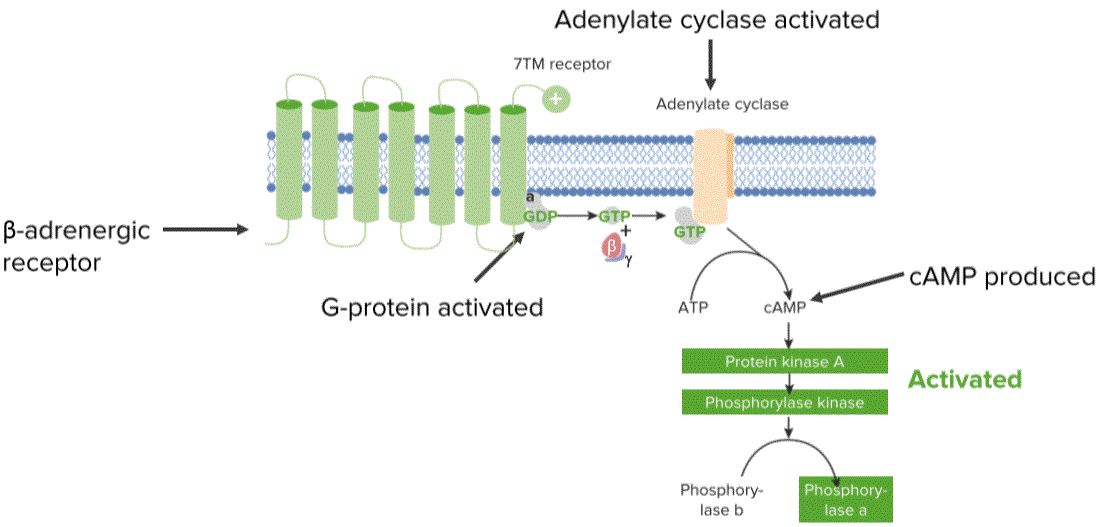
“Protein Communication. Responding to the environment” Image created by Lecturio
Proteins may serve as:
- Transporters
- Channels
- Linkers
- Receptors
Proteins involved in Movement
Another important function of proteins is its involvement in cell movements. There are specialized proteins called motor proteins those bind themselves with the cytoskeleton (system of protein filaments, mainly constituted by actin filaments, microtubules, and intermediate filaments that gives shape and mobility to the cell). Movement along the microtubule length is dependent on the action of motor proteins in it.Type of motor protein varies based on their affinity for the particular filament, their direction of movement and the materials they carry. Many motor proteins carry cell membrane-enclosed organelles of the cells and transport them to appropriate sites, some proteins help the filaments slide over one another causing contraction of cells like in muscle cells, beating of cilia. These proteins utilize energy from ATP hydrolysis to produce movement. Two families of motor proteins are responsible for the cellular movements brought about by microtubules.
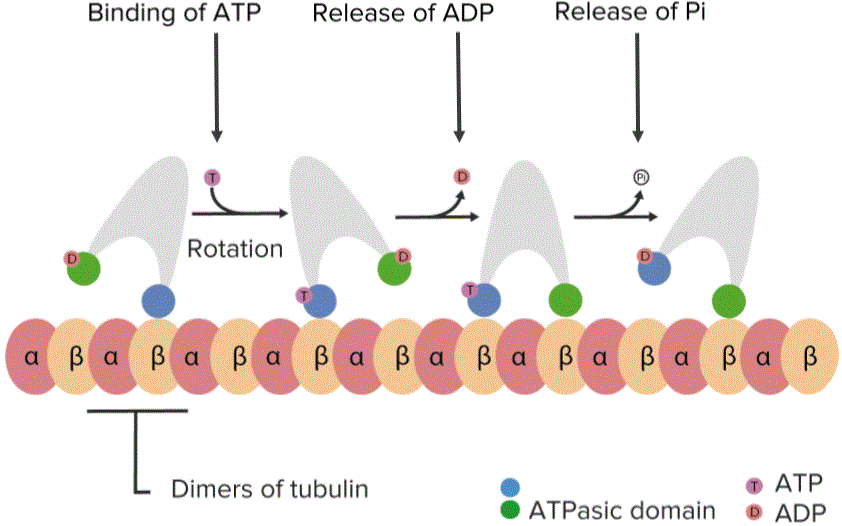
“Proteins involved in movement. Work with microtubules and filaments” Image created by Lecturio
Types of Motor Proteins
These are kinesins, dyneins, and myosins.Kinesins and dyneins move in opposite directions. Kinesins are molecules consisting of two heavy and two light chains. The heavy chains have amino-terminal globular head responsible for binding to both microtubules and ATP. Hydrolysis of ATP provides the energy needed for movement. The two light chains are responsible for binding to the other cell components, such as organelles and vesicles that are transported along microtubules.
Dynein, on the other hand, is a relatively larger molecule also composed of heavy and light chains. The same functions are done by these heavy and light chains. These proteins are responsible for moving organelles toward and away the center of the cells. Some play more specific roles, such as repositioning the Golgi apparatus from the center of the cell to that near the centrosome.
Other important motor proteins are myosin. Myosin is an actin-binding protein. Myosin is considered a prototype of a molecular motor as it is able to convert chemical energy in the form of ATP to mechanical energy. These types of movements can be as complex as the movements in muscle contraction.
An interaction between actin and myosin are responsible for:
- Muscle contraction
- Non-muscle movement
- Cell division
The sliding filament theory explains the possible mechanism in muscle contraction based on the action of myosin and actin. According to this theory, the actin filaments of muscle fibers tend to slide past the myosin filaments during contraction. This was further developed as the cross-bridge model. In the cross-bridge model, actin and myosin form a protein complex called an actomyosin.
The head of the myosin attaches itself to the actin filament forming a cross-bridge between them. Contraction occurs when myosin pulls the actin filaments towards the center of the band, then it detaches itself from the actin, and creates a stroke to bind to a next actin molecule.
Review Questions
The correct answers can be found below the references.1. Which of the following is not a fibrous protein?
- Keratin
- Tubulin
- Elastin
- Collagen
- 1
- 2
- 3
- 4
Comentários
Enviar um comentário