Telencephalon – Language Centers, Structure of the Limbic System and Basal Ganglia
Table of Contents
- Language Centres of the Telencephalon
- Other Symptoms Associated with Damage to the Dominant Hemisphere
- Other Symptoms Associated with Damage to the Non-Dominant Hemisphere
- Structure of the Limbic System
- Structure of the Basal Ganglia
- Parkinson’s Disease
- Huntington’s Disease (Chorea)
- Hemiballismus
- Fibre System of the Telencephalon
- References
Language Centres of the Telencephalon
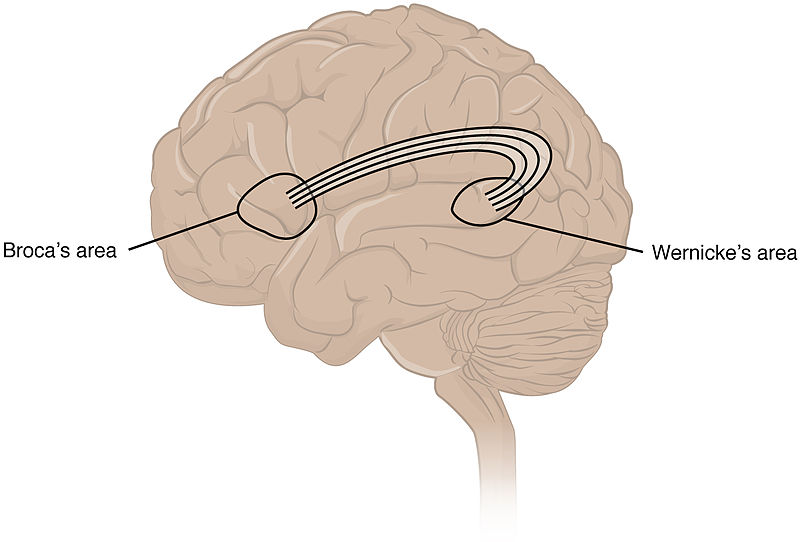
The language centres of the telencephalon include the Broca’s area (Brodman area 44) and the Wernicke’s area (Brodman area 22). The Broca’s area is located in the area of the inferiorfrontal gyrus of the frontal lobe, whereas the Wernicke’s area is located in the centre of the temporal lobe. It is important to note that both language centres are located only on the dominant hemisphere (see above).
Function of the Broca’s Area
The Broca’s area is also referred to as the motor speech centre because it governs the articulation of words and sounds.Studies also suggest that apart from language production it is also associated with action processing and sequencing of motor actions associated with verbal and nonverbal communication. Furthermore, syntax is formed in the Broca’s area.
The Broca’s area also manages the repetition of words via afferents from the secondary auditory cortex, which is located in the area of the temporal lobe and runs around the primary auditory cortex in the form of a horseshoe. In addition to the fibres of the secondary auditory cortex, the fibres of the secondary visual cortex reach the Broca’s area.
Function of the Wernicke’s area
The Wernicke’s area is also called the sensory speech area as it is responsible for the understanding of words and sentences and requests. This area controls the processof phonologic retrieval that is the process of sequencing the sounds of words before they are uttered. The arcuate fasciculus provides a connection between the Wernicke’s area and the Broca’s area.
Speech Disorders due to the Failure of a Language Centre (Aphasia)
Depending on which language centre is affected, the symptoms of aphasia may vary. A common cause for aphasia is a stroke which, in turn, is in most cases (about 80%) caused by ischemia.
Aphasia is defined as an acquired language disorder, with the cause of aphasia being centrally located. Language disorders have to be distinguished from speech disorders (dysarthria), the latter having a variety of causes, such as cerebellar, bulbar or extrapyramidal damages. The main forms of aphasia include Broca’s aphasia, Wernicke’s aphasia, amnesic and global aphasia.
Definition and Symptoms of Broca’s Aphasia (Motor Aphasia)
Broca’s aphasia is the damage of Broca’s area in the language-dominant hemisphere, and is also known as motor aphasia. It is characterised by a disturbance of the fluency of speech which is also slowed down considerably.
Patients usually speak only on request, and the speech is fragmented, telegram-like. Patient speaks in short fragmented sentences that make sense but are uttered with great strain. Small words are often omitted. Patients with Broca’s aphasia may have weakness or paralysis of right side of the body. Patient can easily comprehend others’ speech.
In addition, phonemic paraphasia (see below) occurs. Comprehension, on the other hand, is hardly affected. However, difficulties in understanding long sentences and subordinate clauses can still be observed. In some cases, reading, writing and arithmetic abilities are also affected (alexia, agraphia and acalculia).
Definition and Symptoms of Wernicke’s Aphasia (Sensory Aphasia)
Wernicke’s aphasia is also known as sensory aphasia. The damage is located in the area of the temporal lobe, within the area supplied by the posterior temporal artery.
The main symptoms of Wernicke’s aphasia are an increased output of speech (logorrhea), and phonemic and semantic paraphasias. A patient with phonemic paraphasia has disturbance with phenomic retrieval process and confuses syllables, changes order of syllablesof words or shortens words, whereas a patient with semantic paraphasia confuses entire words. The confused words often come from the same subject area, for example, substituting “arm” for “leg”.
The increased speech output involves, among other things, a making up of new words (neologisms). Unlike in Broca’s aphasia, also speech comprehension is disturbed, which additionally complicates the patient’s ability to communicate. Writing and reading is usually disturbed as well.
Definition and Symptoms of Global Aphasia
In global aphasia, both the Wernicke’s and Broca’s area are affected, as well as the basal ganglia region. This results in a complete loss of speech. Patients express themselves only through sounds and possibly isolated words like “yes” or “no”. Patient can barely understand spoken language. Patient can neither write nor read. Intellectual and cognitive abilities are preserved. This form of aphasia is mainly caused by an extensive media infarct of the language-dominant hemisphere due to stroke or brain trauma. If the basal ganglia are also affected, additional unilateral symptoms, such as a form of hemiparesis, can frequently be observed as well.
This is most severe type of aphasia with chances of improvement dependent upon the damage caused to brain. If damage is not very extensive improvement may occur in 5-6 months otherwise long lasting disability may remain.
Definition and Symptoms of Amnesic Aphasia
In patients with amnesic aphasia, there is not one single damaged area, but rather multiple smaller lesions, in the area of the temporal and parietal lobe of the language-dominant hemisphere.
Clinical presentation of amnesic aphasia involves strong word-finding problems, which are tried to be compensated by phrases and circumlocutions. Spontaneous flow of speech, however, is not affected.
Other Symptoms Associated with Damage to the Dominant Hemisphere
Next to the forms of aphasia described above, damage to the dominant hemisphere may lead to other clinical signs, such as apraxia or agnosia.
Definition of Apraxia
Apraxia describes the inability to adapt behaviour to specific purposes and situations. Based on the clinical appearance, multiple manifestations can be distinguished. These include, for example, the ideomotor and ideational apraxia. Individuals with ideomotor apraxia have damage in the Wernicke’s area and the primary motor cortex, whereas in ideational apraxia,the damages lie in the area of the temporoparietal junction.
Clinical presentation of ideomotor apraxia includes a distortion of aiming movements, movement sequences, as well as facial expressions and gestures. Patients with ideational apraxia are able to perform individual movements correctly, but cannot implement them in a more complex sequence.
Definition of Agnosia
Agnosia makes it impossible to recognise certain things, despite intact senses. Depending on what kind of things cannot be perceived, different forms of agnosia are distinguished.
One form of agnosia is visual agnosia, where patients cannot recognise objects, despite intact vision. When touching the same object, however, they can easily name it.
Further examples include prosopagnosia, which describes the inability to recognise people and their faces, autotopagnosia, which is characterised by the inability to localise parts of the body, and anosognosia, which describes the inability to perceive one’s own body ailments or neurological dysfunctions. Thus, patients who suffer from anosognosia cannot, for instance, recognise a hemiparesis as such.
Other Symptoms Associated with Damage to the Non-Dominant Hemisphere
Damages to the non-dominant hemisphere can cause, inter alia, a so-called neglect syndrome.
Definition of Neglect Syndrome
Neglect is a motor or sensory inattention to one half of the body. A test for detecting such a unilateral neglect is, for example, the clock-drawing test, where a patient is asked to draw a clock face with the clock hands pointing to a certain time. Most patients who suffer from a unilateral neglect will draw all numbers in one half of the clock.
Structure of the Limbic System
The limbic system consists of several components. These are the hippocampus, the parahippocampal gyrus with entorhinal cortex (see above), the cingulate gyrus, the amygdala, and the mammillary bodies.
Function of the Limbic System
The limbic system serves a variety of functions. These include,
- Social cognition
- Control of emotions like fear, rage and tranquility.
- Memory
- Processing of olfactory senses
- Sexual behavior
- Appetite and eating patterns are controlled
- Sleep and dreams- it processes unconscious emotions and conscious thoughts and forms the base of dreams.
- Addiction and motivation pathways are controlled by this system.
- Controls autonomic and endocrinal response to emotions.
Among others, the development of affective and instinctive behavior, the influencing of sexual functions, and the formation of memory contents.
However, the limbic system is not the only system that is responsible for these functions; it is rather an interaction of several brain areas. The individual structures of the limbic system can each be assigned to specific functions. The hippocampus, for instance, is the primary site of memory formation; also behaviour, awareness and motivation originate here.
Alongside the hippocampus, the other components of the Papez circuit are involved in memory formation. These include, for example, the mammillary bodies of the limbic system. Moreover, affective and sexual behaviour are mediated via the mammillary bodies.
Modulation of the autonomic system and development of motivation take place, among other things, in the cingulate gyrus.
The amygdala, which is located in the temporal lobe below the caudate nucleus, plays an important role in the development of behaviours and the storage of memory contents associated with emotions.
The most relevant emotion is here the feeling of fear. Therefore, the amygdala is also referred to as the “fear centre“. If there is damage in the area of the amygdala, it may cause misjudgement of a dangerous situation and, as a result, risky behaviour.
In addition, the amygdala is involved in the modulation of autonomic hypothalamic areas, which may lead to, e.g., an accelerated heart rate in scary situations.
Structure of the Basal Ganglia
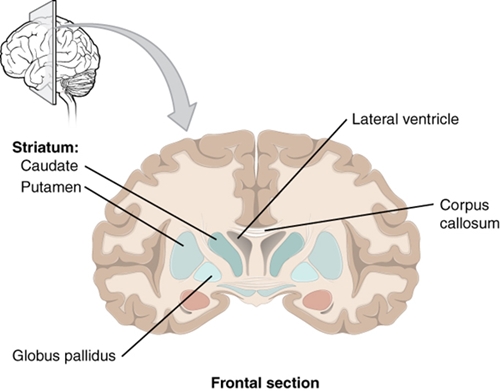
The basal ganglia, which are also known as basal nuclei, include the caudate nucleus, the putamen, and the globus pallidus. They are present in pairs, one on each of the two hemispheres.
An evolutionary particularity of the globus pallidus is that it developed in the diencephalon, but shifted to the telencephalon.
Topography of the Basal Ganglia
The basal ganglia are part of the grey matter of the telencephalon and are located in its inside.
In a cross-section, the head of the caudate nucleus (caput nuclei caudati) is located laterally on both sides of the anterior cornu of the lateral ventricles. The tail of the caudate nucleus (cauda nuclei caudati) lies above the posterior cornu of the lateral ventricle.
Putamen and globus pallidus are located in the cross-section of the brain lateral of the thalamus, with the globus pallidus being more medial.
Between the thalamus and the caput nuclei caudati as medial border, and the globus pallidus and putamen as the lateral border, runs the internal capsule (see below). The white matter of the internal capsule separates the nucleus caudatus from the putamen, and creates a striped appearance, which led to the summarising name “corpus striatum” for the caudate nucleus and putamen.
The corpus striatum is especially well defined in a frontal section. Here, the close relationship of the nucleus caudatus and the ventriculus lateralis becomes apparent. Cranially, the lateral ventricle is adjacent to the corpus callosum. Lateral of the putamen is the capsula externa and the claustrum, which is supposed to be involved in sexual arousal.
Function of the Basal Ganglia
The basal ganglia are part of the extrapyramidal motor system, modulating the effect of voluntary fine motor movements.
Projections of the Basal Ganglia
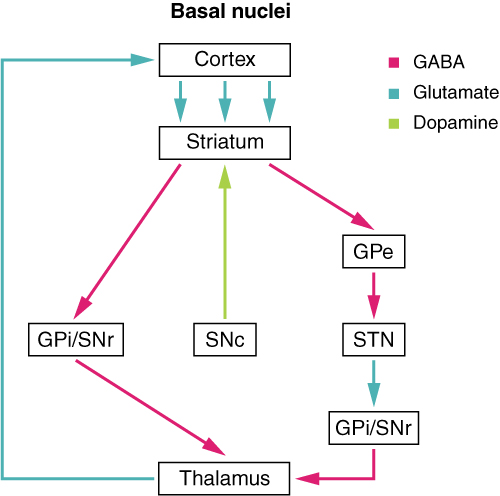
The projections of the basal ganglia that mediate motor information run in a loop, which is called a basal ganglia circuit or motor circuit. Within this circuit, information is transported from the basal ganglia to the premotor cortex of the telencephalon. This information then returns from the primary motor cortex of the telencephalon to the basal ganglia.
The feedback of the information is carried out by the thalamus. In addition to the thalamus and the basal ganglia, the subthalamic nucleus and the substantia nigra also form part of the basal ganglia circuit.
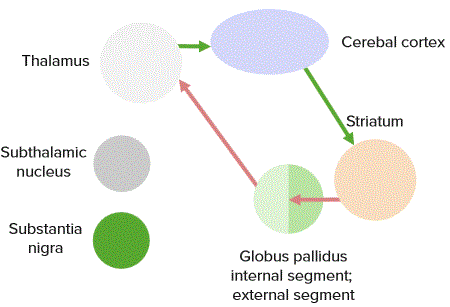
“Direct pathway” Image created by Lecturio
In the basal ganglia circuit, there is a direct and an indirect pathway. In the direct pathway, the putamen projects to the globus pallidus internus and to the reticular part of the substantia nigra. Because of the transmitter GABA, the putamen has an inhibiting effect on the two mentioned projection areas.
This eliminates the inhibitory effect of the globus pallidus and the substantia nigra to the thalamus, and so the latter can act excitatory on the motor cortex. In the indirect pathway, the putamen has an inhibitory effect on the globus pallidus externus, which usually has an inhibitory effect on the subthalamic nucleus. By eliminating this inhibiting effect, the subthalamic nucleus acts with the transmitter glutamate excitatory on the globus pallidus externus, the globus pallidus internus, and the reticular part of the substantia nigra.
The reticular part of the substantia nigra and the globus pallidus internus act inhibiting on the thalamus, so that it does not promote any motor movements. In summary, the direct pathway acts excitatory and the indirect pathway inhibitory on the execution of movements.
Diseases Associated with the Basal Ganglia
The diseases of the basal ganglia are differentiated based on the symptoms into hyperkinetic and hypokinetic disorders. The hyperkinetic disorders include, for instance, Huntington’s disease or hemiballismus; the best known representative of the hypokinetic forms is Parkinson’s disease.
Parkinson’s Disease
The Parkinson’s disease shows damage in the area of the pars compacta of the substantianigra. Death of dopamine-generating neurons occurs which leads to an interruption of the inhibitory effect of the substantianigra on the direct pathway of the basal ganglia circuit. Due to this disinhibition, the indirect pathway becomes more active and inhibits the thalamus, which leads to a reduction of movements.
Definition
Parkinson’s disease is characterized as a movement disorder, because of deranged production of dopamine, a chemical by the neurons.
Clinical features
It is characterized by a typical clinical triad consisting of akinesia, rigidity, and tremor. Sometimes, postural instability is included with the main symptoms as well. The tremor is typically a resting tremor that begins mostly on one side, i.e.it is asymmetric. Poor balance and coordination are marked.
The patient’s gait pattern provides a first indication of the disease. As part of hypo- or akinesia, the gait is characterized by short steps and a forward inflection (propulsion) with a reduced swinging of the arms which is often more pronounced on one side as well. As the disease progresses other symptoms such as sleep disorders, depression, difficulty in chewing, swallowing and speaking appear.
Symptoms are:
- Bradykinesia or akinesia
- Rigidity
- Resting tremor of hands and fingers
- Difficulty initiating movements
- Movements are abnormally slow
- Decreased facial expression
Structural changes and causes:
- Depigmentation of substantia nigra
- Lewy bodies
- Causes include genetics, environment, certain medications, MPTP, and vascular insult
Diagnosis
Diagnosis is made upon the bases of clinical presentation, history, and neurological examination.
Treatment
The treatment of Parkinson’s disease involves different classes of drugs. These include anticholinergics the administration of levodopa, NMDA receptor antagonists, dopamine agonists, MAO-B inhibitors or COMT inhibitors.
Recommendations for the initiation of therapy depend on the biological age of the patient. For patients under 70 years, initiation of therapy with a dopamine agonist is recommended; for patients 70 years and older, it is recommended to use L-dopa (Parkinson guideline of the German Society of Neurology (last updated September 2012)).
However, the treatment regimen has to be individually adjusted to each patient in accordance with his or her dominant symptoms.
Huntington’s Disease (Chorea)
Definition
Huntington’s disease is atrophic lesions in the area of basal ganglia the corpus striatum and, in a later stage of the disease, also in the area of the cerebral cortex. The disease shows an autosomal dominant inheritance and manifests in the 3rd to 5th decade of life.
Pathophysiology/Clinical Features
There is a decrease in the GABA concentration due to the atrophy of the corpus striatum and this leads to an imbalance between GABA and dopamine. Dopamine acts in support of the direct pathway and inhibitory on the indirect pathway of the basal ganglia circuit, which leads to an increased activation of the thalamus and the motor cortex.
The resulting spontaneous movements are noticeable, primarily in the face and distal extremities. These repeated movements lead to an increased caloric consumption and patients often present as cachectic. Food intake is even more difficult when the caudal cranial nerve is affected and chewing and tongue muscles are impaired.
Extrapyramidal movement disorder often follows previous psychopathological changes, which manifest 10 – 15 years before and in the form of, e.g., disturbed motivation, emotion, or psychosis. In the course of Huntington’s disease, the development of dementia is rather common.
Symptoms are:
- Chorea
- Athetosis
- Personality changes
- Dementia
Structural changes and causes:
- Loss of GABAergic, medium size, spiny neurons from striatum
- Ventricular enlargement
- Genetic cause – autosomal dominant
- Excessive CGA repeats
Treatment
The progression of the disease cannot be influenced therapeutically, i.e. there is no causal therapy. However, symptoms can be treated with medication, physiotherapy and ergotherapy. A drug against hyperkinesias is, for example, sulpiride.
Hemiballismus
The cause of hemiballismus is mostly damaging in the area of the subthalamic nucleus. Clinical signs for hemiballismus are suddenly emerging proximal flinging movements.
There is no known causal therapy. Treatment approaches include drugs like neuroleptics, valproic acid, and sometimes benzodiazepines.
Fibre System of the Telencephalon
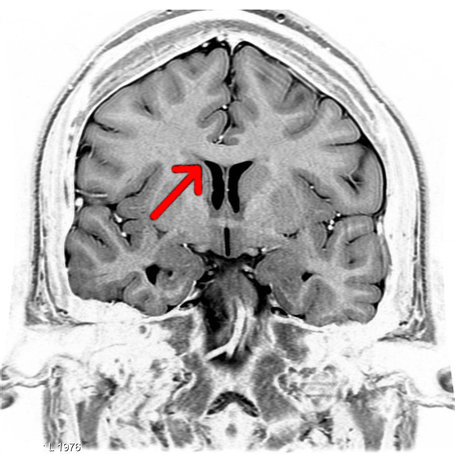
Image: “Coronal T2 (grey scale inverted) MRI of the brain at the level of the caudate nuclei emphasizing corpus callosum.” by Frank Gaillard. License: CC BY-SA 3.0
The fibre system of the telencephalon is divided into three classes, depending on their function. These are the commissural, the association, and the projection fibres.
The task of the commissural fibres is to connect certain areas of both hemispheres with one another, thus enabling an exchange between them. The majority of commissural fibres are summarised in the corpus callosum, which also connects the two hemispheres anatomically.
In contrast to the commissural fibres, the association fibres connect individual parts of the same hemisphere.
Projection fibres connect the cortex with the subcortical areas. These are, e.g., the thalamus or the basal ganglia. The majority of these fibres run through the internal capsule and the externalcapsule.
Structure of the Internal Capsule
The internal capsule is divided into three sections. These are the crus anterius (anterior limb), the crus posterius (posterior limb), and the genu capsulae interna (knee). Specific tracts project through each section, which are also organised somatotypically.
Through the crus anterius runs the frontopontine tract and the anterior thalamic peduncle. In the genu capsula interna, there are parts of the tracts of the dorsal thalamic peduncle. The remaining parts of the dorsal thalamic peduncle’s tracts pass through the crus posterior. In addition, the crus posterius contains the fibrae corticonuclearis, the fibrae corticospinalis, the tractus temporopontinus and the radiatio optica.
Somatotopy of the Internal Capsule
Function of a Commissurotomy (Split-Brain)
A separation of the commissural fibres within the corpus callosum is called commissurotomy. It causes an interruption of the communication between the two hemispheres. This surgical intervention is used in epilepsy treatment as a last resort. The complete sectioning of the corpus callosum prevents generalisation, i.e. the propagation of an epileptic seizure to both hemispheres.
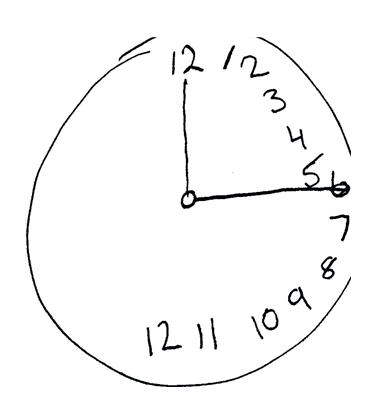


Comentários
Enviar um comentário