Cell-Mediated Immunity
Table of Contents
- Introduction to the Cell-Mediated Immunity
- The T-Cell Receptor Complex
- Helper T-Cell Subsets: Th0, Th1, and Th2 Cells
- Regulatory T-Cells, Cytotoxic T-Cells, Macrophages and Intraepithelial Lymphocytes
- Cytokines, Interferons, Interleukins and Colony Stimulating Factors
- Chemokines, Tumor Necrosis Factors and Transforming Growth Factors
- Therapeutic Blocking of Pathological Cytokines and Therapeutic Cytokines
- Review Questions
- References
Introduction to the Cell-Mediated Immunity
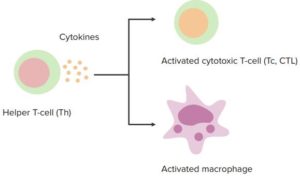
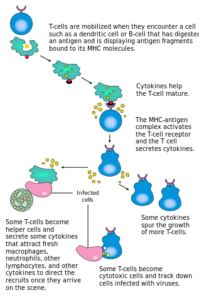
“Cell-Mediated Immunity” Image created by Lecturio
The immunity provided by cytotoxic T-cells and by T helper cell-mediated activation of macrophages. Required to defeat intracellular pathogens which are hidden from the effect of antibody and complement.
The T-Cell Receptor Complex
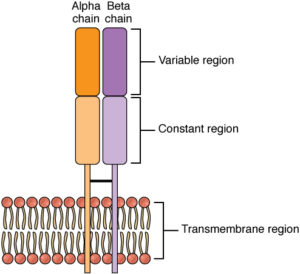
The T-cell receptor (TCR) for antigen is a heterodimer that consists of two transmembrane polypeptide chains called TCR α and β. T-cells with these receptors are called αβ cells. Less commonly, the TCR consists of γ and δ chains, and the T-cells with this type of receptor are called γδ cells.
TCR α and β are covalently linked by a disulfide bridge. Each TCR α and β chain has one Ig-like N-terminal variable (V) region and one Ig-like constant (C) region, with a short cytoplasmic regionand a hydrophobic transmembrane region. The extracellular structure of the TCR is, therefore, similar to the antigen-binding portion (Fab) of an antibody.
TCRs of αβ cells recognize peptide fragments of protein antigens that are bound to major histocompatibility complex (MHC) molecules on the surface of antigen presenting cells, includingdendritic cells, macrophages and B-lymphocytes. The variable region of TCRs binds to peptide antigens, while the constant region binds to signalling molecules.
CD4 and CD8 molecules are expressed on certain T-cell subsets. They function as co-receptors for antigen recognition and activation of the T-cell. In the setting of antigen recognition, CD4 molecules on the T-cell bind to class II MHC molecules on antigen presenting cells, while CD8 molecules bind to class I MHC molecules on antigen presenting cells. Most CD4 T-cells are helper T-cells, while most CD8 T-cells are cytotoxic T-cells, with which they are synonymous.
However, γδ cells do not express CD4 or CD8 molecules on their surfaces. Instead, they recognize non-protein antigen molecules such as bacterial lipoglycans.
Helper T-Cell Subsets: Th0, Th1, and Th2 Cells
Most helper T-cells (Th) express CD4. These cells serve the function of helping other cells with mounting an immune response. They help cytotoxic T-cells by promoting their effector functions, B-cells with antibody production and macrophages with their phagocytic function.
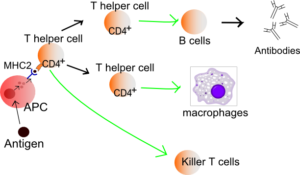
Image: “The T-lymphocyte activation pathway is triggered when a T-cell encounters its cognate antigen, coupled to an MHC molecule, on the surface of an infected cell or a phagocyte.” Derivative work by Hazmat2. License: Public Domain
Th cells also help develop and maintain memory cytotoxic T-cells. There are different subsets of CD4 helper T-cells that have different functions and produce different reactions.
Th0 subset
Type 0 helper (Th0) T-cells are basically naïve T-cells, before they become differentiated into mature helper T-cells.
Th1 subset
Type 1 helper (Th1) cells play a key role in phagocyte-mediated defense by activating macrophages to destroy ingested microbes via lysosomal enzymes, nitric oxide and reactive oxygen species.
The immune reaction mediated by Th1 cells also occurs in delayed type IV hypersensitivity reactions, seen in granulomatous inflammation and many other inflammatory conditions.
Differentiation of CD4 cells to Th1 cells is chiefly stimulated by the cytokines Il-12 and IFN- γ. Microbes infect and activate phagocytes, including macrophages and dendritic cells, which produce IL-12, and this leads to the induction of Th1 cells.
Such microbes include intracellular bacteria, such as mycobacteria and Listeria, as well as parasites, like Leishmania and viruses. Microbes also stimulate NK cells to produce IFN- γ, which induces Th1 cells too, and acts on phagocytes to produce more IL-12.
The development of Th1 cells leads to further Th1 differentiation, as mature Th1 cells produce IFN- γ. IFN- γ stimulates Th1 differentiation, while preventing the differentiation of CD4 cells to the Th2 and Th17 subsets.
Image: “T helper cell function.” by Häggström, Mikael. “Medical gallery of Mikael Häggström 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN 20018762. Public Domain. or By Mikael Häggström, used with permission. – Image:Lymphocyte_activation.png. License: Public Domain
Th2 subset
Type 2 helper (Th2) cells mediate phagocyte-independent immune reactions, which involve eosinophils and mast cells. This type of immune reaction is important against helminthic infectionsand in allergic reactions. The cytokine IL-4 induces the differentiation of Th2 cells, which occurs in response to helminths and allergens.
These antigens cause persistent or repeated stimulation of T-cells that lack a strong inflammatory component, so that Th1 and Th17 responses do not occur, but Th2 responses are induced instead. Th2 cells mediate their actions by IL-4, which causes IgE antibody responses; IL-5, which causes activation of eosinophils; and IL-13.
Regulatory T-Cells, Cytotoxic T-Cells, Macrophages and Intraepithelial Lymphocytes
Regulatory T-Cells
Regulatory T-cells are another subset of CD4 T-cells. Their function is to suppress immune responses and establish self-tolerance. Regulatory T-cells develop mainly in the thymus, with recognition of self-antigen, and peripheral lymphoid organs, in response to self and foreign antigens.
The development of some regulatory T-cells depends on the cytokine TGF-β, while their survival and function depends on IL-2. Dendritic cells in peripheral tissues may also play a role in promoting the development of regulatory T-cells.
Regulatory T-cells suppress immune responses at different levels, including the induction of T-cell development in lymphoid organs and the effector functions of T-cells in peripheral tissues. Other ways that they may suppress immune responses include inhibiting natural killer (NK) cell proliferation and preventing B-cell activation.
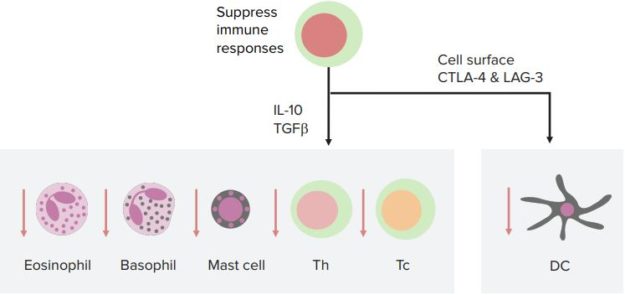
“Regulatory T-Cells” Image created by Lecturio
Cytotoxic T-Cells
CD8 cytotoxic T-lymphocytes have the key function of killing cells that harbor microbes in the cytoplasm. Their other functions include their role in eradicating tumors and the acute rejection of organ allografts.
The differentiation of naive CD8 Т-cells into cytotoxic T-cells occurs in response to antigen presented by dendritic cells. This process may involve CD4 helper T-cells too. With the differentiation and proliferation of naïve CD8 T-cells, effector cytotoxic cells are generated that possess the machinery to kill infected or tumor cells.
Cytotoxic T-cells encompass many lysosomes that contain proteins such as granzymes and perforin. Cytotoxic T-cells also produce cytokines that help with the elimination of intracellular microbes, such as IFN-γ, to activate macrophages.
Macrophages
Macrophages are produced in the bone marrow as immature monocytes. In the blood, they still exist as monocytes, and when they reach the extravascular sites, they become macrophages that survive for weeks or years. Macrophages respond to various stimuli, including cytokines. They feature azurophilic lysosomal granules, which containmyeloperoxidase, lysozyme and acid hydrolases.
Macrophages also produce and secrete other products including a non-specific esterase, neutral proteases such as collagenase and elastase, complement products, prostaglandins, coagulation factors, fibronectin and various cytokines.
Macrophages that are activated in Th1-mediated responses (M1 macrophages) secrete pro-inflammatory cytokines such as IL-1, TNF, IL-6, as well as reactive oxygen species. Macrophages associated with Th2 responses (M2 macrophages) secrete IL-10.
When macrophages are activated in immune responses, this increases their size, their lysosomal enzyme content, their active metabolism and phagocytic ability.
Intraepithelial lymphocytes
Intraepithelial lymphocytes are effector cells of innate immunity. They are, essentially, T-lymphocytes that are present within the epidermis and mucosal epithelium. These lymphocytes express a limited variety of antigen receptors. They provide host defense by cytokine secretion, phagocyte activation and killing infected cells. Intraepithelial lymphocytes are one of the first line defence and are a mixed population of antigen-experienced T-cells.
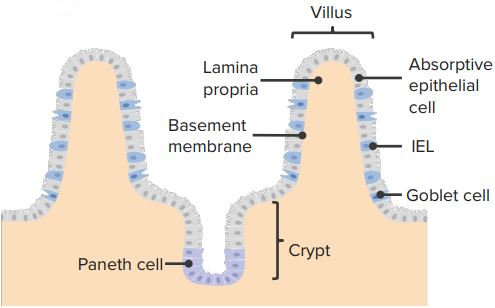
“Intraepithelial-lymphocytes” Image created by Lecturio
Cytokines, Interferons, Interleukins and Colony Stimulating Factors
Cytokines
Cytokines are the messenger molecules of our body’s immune system that serve as mediators of immune responses and inflammation. They constitute polypeptides produced by various cell types.
In innate immune responses, cytokines are chiefly produced by macrophages and dendritic cells. In adaptive immune responses, cytokines are produced by T-lymphocytes, especially CD4 helper T-cells. Cytokines are produced and secreted in response to various external stimuli, such as antigen recognition, microbes and other cytokines.
Interferons
Interferons refer to a subgroup of cytokines that originally received their name for their function in interfering with viral infections. However, it is now recognized that interferons have other important immune functions, too. They are subdivided into type I and type II interferons.
Type I interferons, which include interferon-α and interferon-β, mainly function to prevent viral replication inside cells.
Type II interferon refers to IFN-γ. IFN- γ is the most important cytokine that activates macrophages and is essential in the defense against intracellular microbes. IFN-γ is produced by CD4 Th1 cells, NK cells and CD8 T-cells. It plays a role in both innate and adaptive immunity.
Its role in innate immunity occurs when NK cells secrete IFN-γ in response to infected or stressed cells. In adaptive immunity, IFN-γ is produced by T-cells when antigen recognition occurs.
Interleukins
Interleukins make up one of the most important groups of cytokines; their function is to communicate between leukocytes. Interleukins are a very diverse group, with differing protein structures, as their classification is biological. About 34 interleukins have been identified and described, from IL-1 to IL-35, and the status of IL-14 is in doubt.
Colony stimulating factors
Colony stimulating factors refer to cytokines that stimulate hematopoiesis. Their function is to increase bone marrow production and output of leukocytes and, therefore, replenish leukocytes that are used up in immune reactions and inflammatory processes.
Chemokines, Tumor Necrosis Factors and Transforming Growth Factors
Chemokines
Image: “This is a diagram showing the effect of chemokine concentration gradient on chemotaxis direction. The attracted cell moves through the gradient toward the higher concentration of chemokine.” by Pen1234567. Derivative of image by Kohidai, L. – Own work. Based on File:Chtxphenomen1.png. License: CC BY-SA 3.0
The chemokine family is made up of small, structurally similar proteins (8-10 kD). There are four groups of chemokines based on the arrangement of cysteine residues: CXCL (e.g., IL-8), CCL, CX3CL and XCL (L stands for Ligand)
The word chemokine is short for chemotactic cytokine, which alludes to their chief function. In humans, there are more than 40 chemokines and about 20 chemokine receptors.
Chemokines are cytokines that stimulate the movement of leukocytes and control leukocyte recruitment. Chemokines recruit different subsets of leukocytes, such as neutrophils, lymphocytes or eosinophils, to various sites of inflammation. They activate leukocytes as well; one of their actions is to enhance the affinity of integrins on leukocytes to their ligands on the endothelium.
The other main function of chemokines is the organization of cells in lymphoid organs and other tissues. For example, they are responsible for the segregation of B- and T-lymphocytesin the lymph nodes and spleen.
Chemokines are usually bound to proteoglycans on the endothelium or are present in the extracellular matrix, where they can cause concentration gradients when required. Their actions occur by binding to G-protein coupled chemokine receptors on target cells.
Tumor necrosis factors
Tumor necrosis factor (TNF) is a cytokine produced by activated macrophages, mast cells,endothelial cells and other cell types. TNF is secreted in response to microbial products, products of T-lymphocytes and immune complexes.
TNF causes endothelial activation by stimulating adhesion molecules on the endothelium, leading to enhanced binding and recruitment of leukocytes. It also promotes the production of chemokines and eicosanoids. TNF makes the endothelium more thrombogenic and promotes neutrophil activation and aggregation.
Transforming growth factors
There are two classes of polypeptide transforming growth factors (TGF), TGF-α and TGF-β. TGF-α is produced by macrophages, T-lymphocytes, keratinocytes and various other cells. It stimulates the replication of hepatocytes and epithelial cells.
TGF-β is produced by many cells, including macrophages, T-lymphocytes, platelets, endothelial cells, keratinocytes, smooth muscle cells and fibroblasts.
TGF-β is chemotactic for macrophages, neutrophils, lymphocytes, smooth muscle cells and fibroblasts. It stimulates the synthesis of TIMP (tissue inhibitor of metalloproteinase), angiogenesis and fibroplasia. TGF-β inhibits matrix metalloproteinase (MMP) production and keratinocyte proliferation. It also plays a role in regulating cytokines, including integrin expression.
Therapeutic Blocking of Pathological Cytokines and Therapeutic Cytokines
Cytokine inhibitors and their applications
Therapeutic cytokines and their application
Review Questions
The correct answers can be found below the references.
1. Which of the following T-cell subsets is important against helminthic infections?
- Th0 cells
- T regulatory cells
- Th1 cells
- Th2 cells
- Th17 cells
2. Which cytokine is inhibited by the monoclonal antibody infliximab?
- IFN-γ
- IL-14
- TNF-α
- IL-8
- G-CSF

Comentários
Enviar um comentário