Cell Surface Receptors: Types & Downstream Mechanisms
Table of Contents
Image: “Solid cell nests” by
Ken Berean. License: CC BY-SA 2.0
Receptors
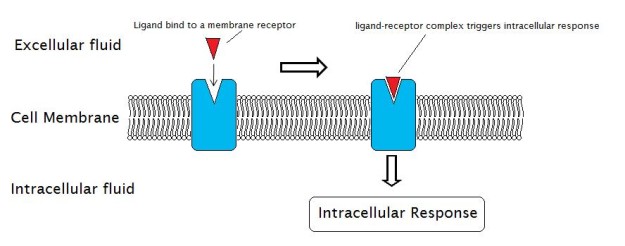
Image: “External Reactions and the Internal Reactions of Receptors” by Laozhengzz. License:Public Domain
A receptor is a molecule that receives signals (chemical or hormonal) from outside the cell and is usually located on the cell surface. Receptors are proteins whose conformational change produced by the attachment of the signaling protein induces a chain reaction within the cell that leads to many possible changes, including cell death.
The molecule that binds to the receptor is called ligand and can be a peptide, a hormone, neurotransmitter, drug or a toxin etc. Each receptor possesses two functional domains: the recognition domain which binds ligands such as hormones, and the coupling domain which is involved in signal transduction.Signal transduction includes the events within the cell that occur in response to a signal following the binding of a ligand to the receptor outside of the cell, on surface of the cell membrane.
Cell Surface Receptors
Cell surface receptors are found on the outer surface of the cell membrane and bind to the extracellular signaling molecule (ligand) to undergo signal transduction. Cell surface receptors in the form of trans-membrane proteins are embedded intrinsically into the plasma membrane and play an essential role in maintaining communication between the processes inside the cell and various types of extracellular signals.
Such extracellular signals (ligands) include hormones, cytokines, growth factors, neurotransmitters, lipophilic signaling molecules such as prostaglandins and cell recognition molecules. When any of these ligands binds to the receptor, it triggers a conformational change that initiates the intracellular signaling pathway. Note that each ligand has its own specific surface receptor.
Additionally, cell surface receptors are specific to individual cell types and thus are also known as cell-specific proteins. They regulate a multitude of biological pathways required for cell growth, survival, differentiation and proliferation etc..
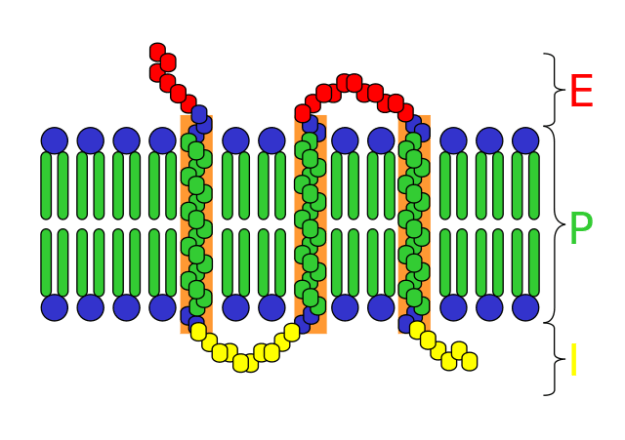
Image: “A schematic of a transmembrane receptor; E = extracellular space P = plasma membrane I = intracellular space” by Mouagip. License: CC BY-SA 3.0
A cell-surface receptor has the following significant components/domains:
- Extracellular domain: an external domain which binds ligands and is exposed to the outer surface of the cell; also known as recognition domain
- Membrane-spanning region: made up of hydrophobic protein molecules
- Intracellular domain: is in contact with cell cytoplasm and is also called coupling domain
Several factors govern the properties of these regions including size and extent of domains, which may vary according to the type of the cell surface receptor. Cell surface receptors are responsible for most of the signaling in multicellular organisms.
Cell Surface Receptors: Types
Cell-surface receptors are generally classified into the following categories:
- Ligand-gated ion channel-linked receptors
- Enzyme-linked receptors
- G-protein-linked receptors
Ion channel-linked receptors
Ion channel-linked receptors are referred to as ionotropic receptors and are responsible for the transduction of chemical signals across the cell in response to the binding of a chemical messenger (a ligand such as a neurotransmitter). They regulate the opening or closing of ion channels that allow ions like Na+, K+, Ca2+, or Cl−, etc. to enter the plasma membrane.
Ion channels are pore-forming proteins also referred to as cell-membrane bound receptors. They are mostly found on synaptic structures and are involved in neuronal activities. Ions pass down their electrochemical gradient through ion channels without requiring ATP or metabolic energy. Ion channels are an important component of the nervous system because when activated by neurotransmitters, they mediate conduction across nerve synapses.
Ion channels also play a vital role in exerting cellular response to toxins and venoms. Various biological processes involving fast changes in cells such as the contraction of cardiac, skeletal, and smooth muscles, activation of T-cells, and the release of hormones are also mediated through ion channels.
Downstream mechanism
When a ligand binds to ion-channel linked receptors, the extracellular domain of the receptor undergoes changes in its configuration which open a channel across the plasma membrane. This allows specific ions (such as Na+, Ca2+, H+, and Mg2+, etc.) or other key molecules to pass through the open channel. The membrane-spanning region of these receptors helps to form a channel through which ions can pass.
Ligands that bind to ion channel-linked receptors include neurotransmitters and peptide hormones and the passing molecules are ions such as sodium (Na+) and potassium (K+).
The amino acids that occupy the membrane-spanning region of ion-channel receptors are hydrophobic in nature, which makes it easier for the phospholipid fatty acid chains that form the core of cell membranes to interact with them. On the other hand, amino acids lining the inside of these channels are hydrophilic, thus allow easy passage of ions and water. The ion channels (or pores) remain open only for a limited time. After that, the ligand is dissociated from the receptor, now available to bind again with a new ligand.
Non-chemical stimuli can also cause the ion channel receptors to act in the same way. Such non-chemical stimuli include changes in electrical charge or mechanical disturbances of the membrane.
Enzyme-linked receptors
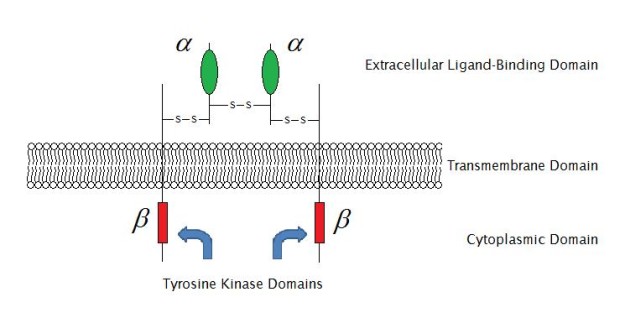
Image: “Enzyme-linked receptor structure (structure of IGF-1R) ” by Laozhengzz. License: Public Domain
Enzyme-linked receptors are typically single-pass transmembrane proteins which act as enzymes or are associated with enzymes. Enzyme-linked receptors have both an extracellular binding site for chemical signaling and an intracellular domain whose catalytic action is controlled by the binding of an extracellular ligand and are thus also called catalytic receptors. There are six types of enzyme-linked receptors:
- Receptor tyrosine kinases – phosphorylate specific tyrosine residues on specific intracellular signaling proteins (EGFR). They bind to polypeptide growth factors which are responsible for controlling cell proliferation and differentiation
- Tyrosine-kinase-associated receptors – these enzymes associate with intracellular proteins that have tyrosine kinase activity (Cytokines)
- Receptor-like tyrosine phosphatases – remove phosphate groups from tyrosines of their target intracellular proteins.
- Receptor serine/threonine kinases – phosphorylate specific serines or threonines on associated gene regulatory proteins
- Receptor guanylyl cyclases – directly catalyze the production of cyclic GMP in the cytosol (Natriuretic peptides receptor)
- Histidine-kinase-associated receptors – activate a 2-component signaling pathway where the kinase phosphorylates itself on histidine residues(autophosphorylation) and then immediately transfers the phosphate to a second intracellular protein. Not present in animal cells
Downstream mechanism
When the enzyme-linked receptor or an enzyme associated with this type of receptor is activated, a multitude of intracellular pathways are efficiently regulated. The intracellular domains of enzyme-linked receptors are associated with an enzyme or directly interact with it. Or, the intracellular domain of the receptor itself may be the enzyme.
Irrespective of large intracellular and extracellular domains of enzyme-linked receptors, a single alpha-helical region of the peptide chain is responsible for forming the membrane-spanning region of enzyme-linked receptors. Activation of the enzyme takes place as a result of the signal transmitted through the channel after a ligand binds to the extracellular domain of receptor.
In most cases, activation of the enzyme takes place because the receptor is dimerized upon binding of a ligand. The activated enzyme leads to an intracellular cascade of events executing the response.
G-protein linked receptors
G-protein-coupled receptors (GPCRs) are the largest cell surface receptors: 7 trans-membrane proteins in the plasma membrane. GPCRs are responsible for the activation of trimeric membrane-bound G-proteins (GTP binding proteins) which subsequently activate either an ion channel (effector) or an enzyme in the cell membrane.
G-proteins or GTP-binding proteins function as an intermediate transducer molecule that plays a vital role in regulating intracellular functions through a secondary mechanism which is in turn activated by G-protein coupled receptors. Many different types of G-protein-coupled receptors are known, such as the acetylcholine (Ach) receptor, β-adrenergic receptor, metabotropic glutamate receptors, certain olfactory receptors, receptors for peptide hormones, and rhodopsin (a photosensitive receptor).
Many neurotransmitters, neuropeptides and peptide hormones, etc. can activate G-protein coupled receptors. GPCRs are responsible for targeting various signaling pathways including sensory perception such as sight, taste, smell, and pain sensations.
Downstream mechanism
When the alpha subunit is reversibly bound to GDP, the receptor is inactivated. However, the GDP can be exchanged for GTP with facilitation by a guanine-nucleotide exchange factor (GEF) and cause activation ofthe receptor.
After a ligand binds to G-protein-linked receptors, of the G-protein is activated. The activated G-protein in turn activates either an ion channel (effector) or an enzyme in the cell membrane. A cyclic series of events takes place as a result of cell signaling with G-protein-linked receptors. GPCRs are among the most important cell surface receptors, and almost half of the drugs exert their action by modifying these receptors.
Review Questions
The correct answers can be found below the references.
1. The passage of ions through the pores of ion channel-linked receptors is a process governed by…
- …ATP.
- …metabolic energy.
- …active diffusion.
- …passive processes.
2. Tyrosine kinases are associated with which class of receptors?
- Steroid hormone receptors
- Enzyme linked receptors
- Ion-channel receptors
- G-protein coupled receptors
3. After a ligand binds to ion-channel linked receptors, a change in configuration takes place which allows the ions to pass through a pore. In which region of which receptor does this conformational change take place?
- Extracellular domain
- Intracellular domain
- Hydrophobic region
- Coupling domain
Comentários
Enviar um comentário