Physiology of the Heart
Table of Contents
- Introduction to the Physiology of the Heart
- Anatomical Composition of the Heart
- Mechanics of the Heart
- Excitation and Electrical Conduction Systems
- Cardiac Action Potential
- Electromechanical Coupling
- Hyperkalemia and Hypokalemia
- ECG – Electrocardiography
- Control of Cardiac Activity
- Blood and Oxygen Supply of the Heart
- Review Questions
- References
Image: “hjertemedisin_ergometersykkel_ekg_190911_web-4” by Universitetssykehuset Nord-Norge (UNN). License: CC BY-ND 2.0
Introduction to the Physiology of the Heart
Being the central element of blood circulation, the heart is responsible for maintaining uniform blood circulation in the body. It also participates in the regulation of the blood pressure: it responds to the changes in blood volume by adjusting contractility and frequency. Produced in the right atrium, the ANP (atrial natriuretic peptide) hormone regulates the body’s water and salt levels when the atrium is stretched – more water will be then excreted through the kidneys in order to reduce blood volume and blood pressure.
The functions of the heart:
- Maintains uniform blood circulation
- Contributes to blood pressure regulation (frequency, contractility, hormonal)
Anatomical Composition of the Heart
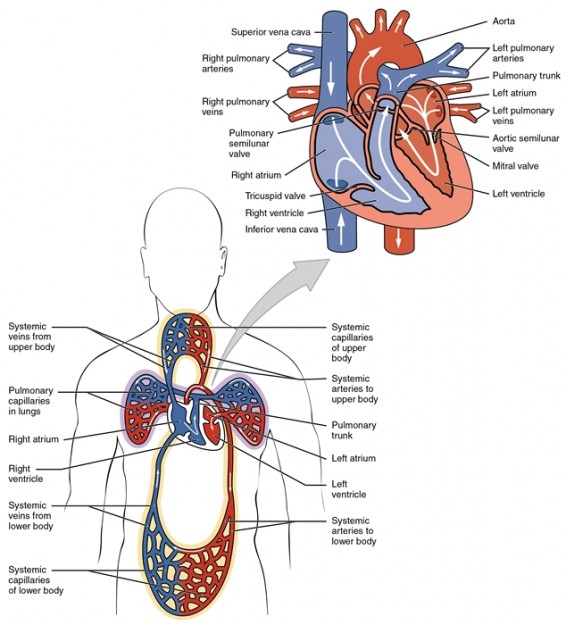
Before going into the depths of physiology, we should first recapitulate the structure of the heart. The heart is a muscular hollow organ that is situated in the mediastinum in the chest. It has two chambers (ventricles) and two atria (sg. atrium). Of the four valves, two separate the aorta and the pulmonary artery from the left and right ventricle respectively. The ‘right’ heart supplies the lungs with blood, while the ‘left’ heart supplies the rest of the body.
The heart consists mostly of muscle tissue (myocardium). There is also a separating layer of connective tissue between the atrium and the ventricle called cardiac skeleton. The heart is lined on the inside with endocardium and covered on the outside with epicardium. The solid capsule surrounding the heart is called pericardium (lat. endo, myo, epi, peri). The upper part of the heart where major vessels are attached to it is called the base of heart (basis cordis) (it is not the bottom part, as one might think), and the downward opening part is called apex (apex cordis).
Blood Supply of the Heart
The heart itself is supplied with blood by the two major coronary arteries – the left and right coronary arteries. Both coronary arteries originate from the aorta (aortic sinus), right behind the aortic valve. The heart supplies its fibers with a part of the expelled oxygen-rich blood.
Excitation and Electrical Conduction Systems of the Heart
The excitation and conduction of the heart are possible because of the cardiac muscle. This muscle belongs to the group of striated muscles and in contrast to skeletal muscles, it is innervated only vegetatively and thus is not arbitrarily controllable.
Heart beating requires muscle contraction. There are centers of special muscle cells in the myocardium that can spontaneously generate electrical excitation – so-called pacemaker cells. These cell areas are therefore referred to as excitation and electrical conduction systems and the remaining muscle cells stimulated by this contraction are called working myocardium.
Pacemaker cells have no resting membrane potential but spontaneously depolarize. They generate action potentials towards the other heart muscles at regular intervals (in case of pacemaker cells in the sinus node, 60–80 impulses per minute). The sinus node is therefore often called the primary pacemaker.
In case of a sinus node dysfunction (e.g., due to a myocardial infarction in this area caused by a vascular obstruction preventing oxygen supply), the cardiac rhythm of the atrioventricular (AV) node sets the rate of 40–55 beats per minute. As a tertiary pacemaker, the conduction system(bundle of His, His-Tawara bundle, Purkinje fibers) in the ventricle only has a rhythm of between 25–40 beats per minute.
Structures of the Heart at a Glance
Anatomy
- Endocardium, myocardium, epicardium, pericardium
- Right ventricle
- Left ventricle
- Right atrium
- Left atrium
- Heart valves (mitral, pulmonary, tricuspid, and aortic valves)
- Base and apex of the heart
Supply
- Left and right coronary arteries
Excitation and electrical conduction systems
- Sinus node
- Atrioventricular node (AV-node)
- Bundle of His
- His-Tawara bundle
- Purkinje fibers
Prof. Joseph Alpert describes the anatomical structures of the human heart in the lecture “Anatomy of the Heart”.
You can watch the full lecture here: Cardiology.
Mechanics of the Heart
Cardiac Cycle
The cardiac cycle consists of systole, during which blood is expelled from the heart, and diastole, during which the heart fills passively with blood via muscle relaxation. The systole is divided into the contraction phase and the ejection phase, while the diastole is divided into the relaxation and filling phases.
If during the filling phase, the pressure inside the ventricles exceeds the pressure inside the atria, the atrioventricular valves (mitral and tricuspid) close. During the contraction phase, all cardiac valves are closed when the ventricles begin to contract, which is why this is referred to as isovolumetric contraction (“iso” means equal, referring to the constant volume during the contraction). The pressure inside the heart increases.
The ejection phase begins with the opening of the semilunar valves (aortic and pulmonary valves) after the ventricular pressure exceeds the pressure inside the aorta and the pulmonary trunk. This is auxotonic contraction. This means that ventricular pressure increases and, at the same time, ventricular volume decreases. This pressure-volume change can be physically described with Laplace’s law:
K = Ptm * (r / 2 * d)
The following applies: K = wall tension, Ptm = transmural pressure, r = ventricle radius, d = wall thickness. With the ventricle volume and hence ventricle radius decreasing, the thickness of the wall increases. It, therefore, follows that with constant wall tension, transmural pressure increases, which allows auxotonic contraction. During systole, the valve plane is pulled toward the apex due to the attachment of the pericardium to the diaphragm.
The relaxation phase starts with the closing of the semilunar valve. The relaxation of the vertical myocardium is isovolumetric. If the ventricle pressure falls below the pressure within the atria, the AV valves open.
During diastole, the valve plane returns to its initial position which makes the AV valves cover the blood volume of the atria, thus reaching almost maximum filling of the ventricles (80–90 %). This process is called valve plane mechanism. Fürthermore, the atrial contraction causes the filling of the ventricles during the filling phase (10–20 %). After the AV valves close, a new cycle begins.
Excitation and Electrical Conduction Systems
All cells of the working myocardium are attached to each other with gap junctions and thus create the so-called functional syncytium. The electrical excitation generated by the pacemaker cells spreads to the cells of the atria. Due to the connective tissue of the cardiac skeleton, there is an electrical insulation of sorts between the atria and the ventricles: excitation can be conducted on the ventricles only through the cells of the AV node that is located there.
At this point, the AV node plays the role of a kind of ‘brake’ – its cells slow down the further conduction of the excitation, so that the ventricles contract later than the atria. This is what makes for the typical pumping rhythm of the heart that can be observed in images. The muscle fibers of the conduction system (bundle of His, His-Tawara bundle, Purkinje fibers) then conduct the potential very quickly through both ventricles causing them to contract evenly.
The conducting function of the AV node also brings about that in the event of a third-degree atrioventricular block, the ventricles and atria pump independently from each other – represented in EKG as separate curves with independent rhythms. Since the intrinsic rhythm of the ventricular pacemaker is so low that the contraction of the chambers might fail, this diagnosis makes a pacemaker absolutely necessary! If conduction slows down or is temporarily interrupted, this is called first-degree or second-degree atrioventricular block.
Cardiac Action Potential
The action potentials of pacemaker cells are significantly different from those in working myocardium. This topic is worth your attention because it is a popular exam subject.
Pacemaker Cells
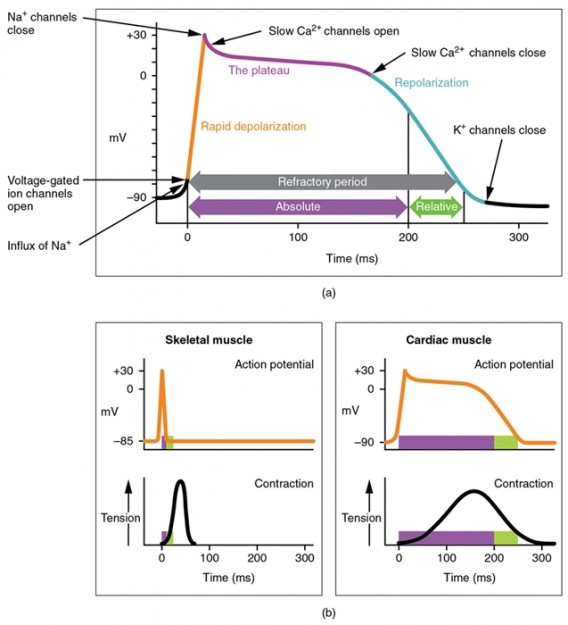
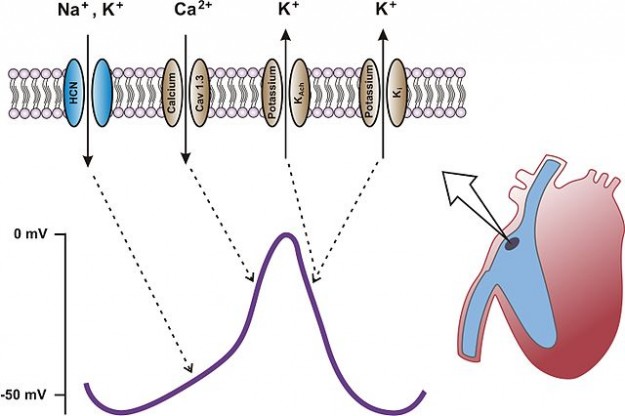
As described above, pacemaker cells are capable of autonomic excitation of the working myocardium since they can depolarize spontaneously. After the excitation of the cells and repolarization, the membrane potential does not become a static resting potential (as is the case with other cell types); rather, the cell slowly begins to depolarize again. This process is called slow diastolic depolarization (SDD) because it takes place during diastole when the heart muscles are not contracting.
A special, voltage-gated ion channel in the pacemaker cells (called HCN channel or ‘funny channel’) makes this possible. These cells repolarize up to -60 mV, resulting in slow diastolic depolarization during which the membrane potential becomes positive again due to the influx of sodium and potassium cations. The threshold potential (potential for opening) of the HCN channel is -40 mV.
Once this threshold is reached, the channels open and Ca2+ enters the cell. The rapid increase in Ca2+ conductivity triggers an action potential. Voltage-gated potassium channels allow potassium flow out of the cell, the membrane potential becomes negative and the cell repolarizes. Once -60 mV is reached, the slow depolarization starts a new cycle.
Important: The duration of an action potential varies depending on the excitation system. It is presumed that the reason lies in a different number of channels in the cells. In the Purkinje fibers, it lasts the longest
Bild: “scheme of heart with sinus node, action potential and contributing ion channels” von Franz Hofmann. Lizenz: CC BY-SA 3.0 DE
Working Myocardium
In some important aspects, the action potential of the working myocardium is different from the one in pacemaker cells.
In contrast to the pacemaker cells, the muscle cells of the working myocardium have a stable resting membrane potential (-85 mV) during diastole. It is sustained by the potassium flux through the voltage-gated potassium channels.
If an electric impulse is received from the pacemaker cells at this point, more and more potassium channels will close and the membrane potential will become more positive. Once the threshold potential of -65 mV is reached, voltage-gated sodium channels open up, sodium flows rapidly into the cell – an action potential occurs. The repolarization initially begins in the voltage-gated calcium channels (L-type Ca2+) and finally reaches resting membrane potential by reopening the potassium channels. The in-flowing calcium is also an important signal transmitter for the electromechanical coupling, ensuring that cells are not only excited but also contracted.
Action Potential in the Heart at a Glance
Action Potential in Pacemaker Cells:
- No stable resting membrane potential
- Slow diastolic depolarization
- Depolarization through calcium influx
- Varied duration
Action Potential in the Working Myocardium:
- Long duration (300 ms)
- Resting potential/plateau phase
- Extremely rapid depolarization
- Depolarization through sodium influx
Electromechanical Coupling
The electromechanical coupling describes the transformation of an incoming electrical signal (in the form of action potential) into a mechanical contraction of the cardiac muscle. It is similar to the electromechanical coupling in the skeletal muscle, but they differ fundamentally on one key point:
In the cells of the cardiac muscle, calcium efflux from the sarcoplasmic reticulum is responsible for the intracellular increase in calcium, which is necessary for the contraction of the cardiac muscle. In comparison, in the skeletal muscle, a mechanically coupled receptor in the cell membrane (DHPR: dihydropyridine receptor) causes the increase of the calcium levels in the interior of the cells during depolarization.
Hyperkalemia and Hypokalemia
Knowing about the influence of the electrolyte concentration in the blood on the heart rhythm is essential not only for an examination but also for clinical practice. The concentration of potassium in and around the cardiac muscle affects the conductivity of the potassium channels and hence the excitation in the cardiac muscle.
An elevated potassium level (hyperkalemia, above 5.0 mmol/L) can lead to the reduced excitability of the working myocardium, cardiac arrhythmia and, in the worst case scenario, cardiac arrest. Potassium deficiency (hypokalemia, below 3.6 mmol/L) also leads to cardiac arrhythmia but in a ‘more active’ way: symptoms include ventricular extrasystoles and ventricular fibrillation.
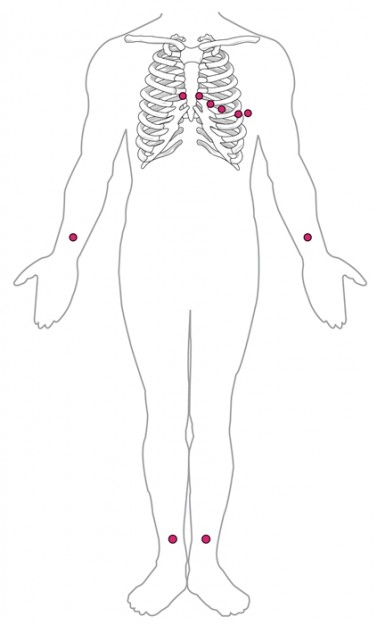
ECG – Electrocardiography
Using electrodes, ECG can record electric fields on the skin surface of the body. They are created by the transfer of charges during the spread of electrical excitation in the heart and are represented in the ECG by the differences in potential. The ECG waves only provide information about the spread of excitation and involution in the myocardium (but not about its mechanical activity).
Vector Theory
The mathematical vector that describes the strength and direction of the electric field points from the excited to the unexcited cells.
According to Einthoven, the limb leads are bipolar.
According to Goldberger, the limb leads are unipolar. With the help of an operational amplifier circuit, two electrodes are connected to an indifferent electrode at high resistance level.
This knowledge allows us to understand the basics of the voltage curve in an ECG. The current resulting vector is projected onto the respective limb lead. If a vector is perpendicular to the direction of a limb lead, the potential difference measured in this limb lead is 0 mV, since the scalar product (the projection of one vector onto another) is 0.
If a vector is parallel to the limb lead, its total value is measured as potential difference.
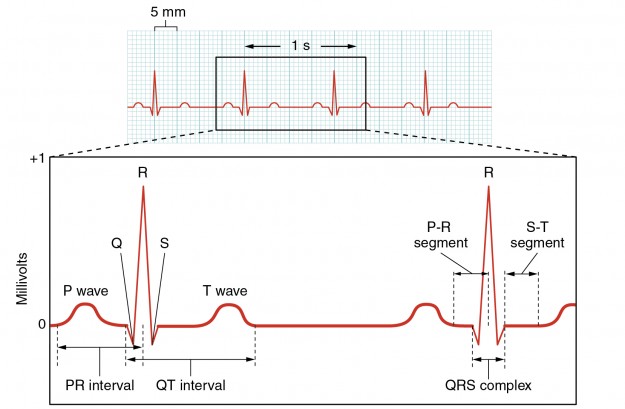
ECG Curve
Every student should know the parts of the ECG curve by heart. Memorizing the description and significance of each part will later help students to learn the interpretations of pathological ECGs.
P wave: excitation spreading in the atrial myocardium
PQ interval: AV node conduction (200 ms)
QRS complex: excitation spreading in the ventricular myocardium
R peak: ventricular excitation spreading from the base to the apex (assessment of the heart’s electrical axis)
S-T segment: ventricles are excited
T wave: repolarization of the ventricular myocardium
QT interval: approximate duration of an action potential (300-400 ms)
Isoelectric line: fully excited or unexcited myocardium (zero potential)
Most common ECG pathologies
First-degree atrioventricular block – PQ interval is extended (200 ms).
Second-degree atrioventricular block – not every QRS complex is followed by a P wave.
Third-degree atrioventricular block – P waves and QRS complex appear independently, but regularly, from each other.
Ventricular extrasystoles – deformed and broadened, ‘formless’ QRS.
Myocardial infraction – elevation of ST segment.
Blockage of the ventricular conduction system – broadened QRS, heart’s electrical axis altered.
Control of Cardiac Activity
Frank-Starling Mechanism
The heart can adapt to short-term changes in pressure and volume via the Frank-Starling mechanism (intracardiac) and via vegetative innervation (extracardiac).
Effects of the Autonomic Nervous System
The autonomic nervous system regulates the performance parameters of the heart. Via the distribution of their neurotransmitters, the sympathetic and parasympathetic nervous systems activate signaling pathways in the myocardium, which affect both pacemaker cells and the working myocardium and influence the parameters of the cardiac output. The most important of them are:
- Heart rate (chronotropic)
- Force of contraction (inotropic)
- Conduction speed (dromotropic)
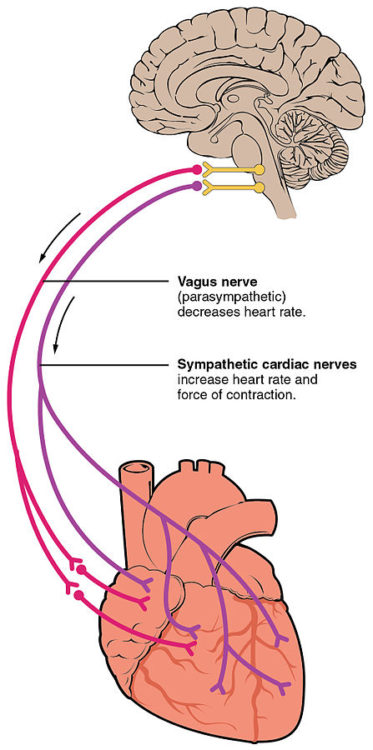
Image: “Autonomic innervation of the heart” by OpenStax College. License: CC BY-SA 3.0
The parasympathetic nervous system regulates cardiac activity via the neurotransmitter acetylcholine, having an inhibiting effect. Muscarinic (acetylcholine) receptors can be found in the atria; the parasympathetic nervous system has almost no effect on the ventricles. Bound to the muscarinic receptors (M2), the neurotransmitter activates the inhibiting G protein (Gi protein).
As a result of the signaling cascade, potassium channels open up – as was mentioned earlier, the slow diastolic depolarization of the pacemaker cells thereby extends; the action potential occurs after some delay; the heart rate decreases.
The sympathetic nervous system raises the level of activity via the neurotransmitters noradrenaline and – to a smaller extent – adrenaline. The ventricles are innervated only by the sympathetic fibers. The neurotransmitter is bound to beta-1 adrenergic receptors and thus activates a stimulating G protein (Gs protein). As a result, sodium and calcium channels open even wider. The increased sodium conductivity leads to faster action potentials, the heart rate increases. The additional calcium flux supports the electromechanical coupling; the force of contraction also increases.
Autonomic Nervous System of the Heart at a Glance
The effect of the sympathetic and parasympathetic nervous systems on the heart is an essential examination topic. If a student devotes some time to look up the signaling pathways of the different receptors in a physiology textbook, they will later understand pharmacology more easily: some cardiotropic substances have their application point in this system. Beta-blockers (e.g., metoprolol) are probably the most common – they reduce frequency and contraction force and are used, for instance, for treating diseases like coronary heart disease and arterial hypertension.
Blood and Oxygen Supply of the Heart
Coronary Arteries
The cardiac muscle needs continuous and sufficient supply of oxygen in order to maintain the same level of contraction force. Our heart requires about 10% of the entire amount of oxygen in the body (10–50 ml/min depending on rest or stress). Cardiac tissue uses the oxygen transported by blood very efficiently – about 70% (compared to 25% in the rest of the body). This means that increased oxygen demand during a physical activity should be compensated by the increased blood flow in the coronary arteries because the usage of oxygen cannot increase significantly. The coronary circulation, however, can be increased by vasodilation up to five times.
The left and right coronary arteries split and form numerous anastomoses with each other. In case of thrombosis or stenosis in one of the coronary arteries, these collateral circulations cannot sufficiently supply the myocardium. It leads to ischemia (oxygen deficiency) and, in the worst case scenario, to necrosis of the heart tissue – myocardial infraction.
Review Questions
The correct answers can be found below the references.
1. The excitation in the working myocardium is not characterized by:
- slow diastolic depolarization.
- a stable resting membrane potential.
- a threshold potential of -65 mV.
- depolarization via sodium influx.
- the long duration of an action potential (300 ms).
2. During which phase of the cardiac activity occurs an auxotonic contraction?
- Contraction phase
- Ejection phase
- Relaxation phase
- Filling phase
- None of the above
3. The elevation of ST segment can be observed in ECG in case of:
- third-degree atrioventricular block.
- first-degree atrioventricular block.
- ventricular extrasystoles.
- myocardial infarction.
- ventricular fibrillation.
Comentários
Enviar um comentário