Reproductive Pharmacology
Table of Contents

Image: by wr52351. License: CC BY-ND 2.0
Definition of Sex Hormones
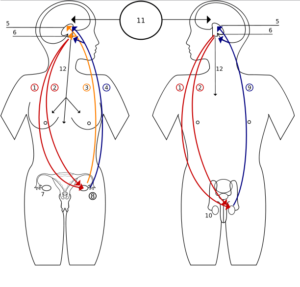
Image: “Hormones feedback. 1 Follicle-Stimulating Hormone – FSH
2 Luteinizing Hormone – LH
3 Progesteron
4 Estrogen
5 Hypothalamus
6 Pituitary gland
7 Ovary
8 Pregnancy – hCG (Human chorionic gonadotropin)
9 Testosteron
10 Testicle
11 Incentives
12 Prolactin – PRL” by Shazz, License: CC BY-SA 3.0
2 Luteinizing Hormone – LH
3 Progesteron
4 Estrogen
5 Hypothalamus
6 Pituitary gland
7 Ovary
8 Pregnancy – hCG (Human chorionic gonadotropin)
9 Testosteron
10 Testicle
11 Incentives
12 Prolactin – PRL” by Shazz, License: CC BY-SA 3.0
Sex hormones are derived from steroid rings which originate as cholesterol. Males are predominant androgen producers while females are predominant estrogen and progesterone producers.
Sex hormones play a distinct role in each gender’s sexual development
with androgens influencing sperm production in men. In women, estrogen
and progesterone regulate the menstrual cycle and are necessary for
maintaining pregnancy. Testosterone is produced in the male testes and by the adrenal gland. Estrogen is produced in its most potent form by the female ovaries.
Physiology of Sex Hormones
Estrogens, progesterone and androgens act on the genomic DNA to
influence gene expression. The expression changes lead to modifications
in cellular function, growth and development.
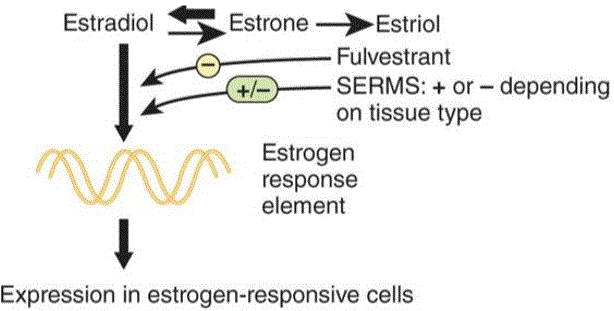
Physiology of estrogen
The predominant female sex hormone is estrogen. Estrogen exerts its
function when it binds to estrogen receptors, which can influence
metabolism and transcription factors. Estrogen is produced in several
locations: the ovary, which makes 17β estradiol; placenta, which produces estriol; and in adipose tissue, which produces estrone from the enzyme aromatase.
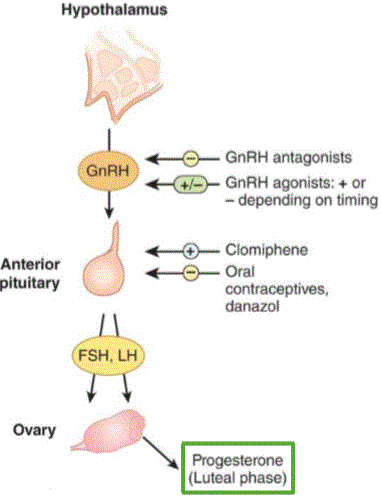
In the ovary, estrogen is produced in the two-cell method. The hypothalamus produces gonadotropin releasing hormone (GnRH) in a pulsatile manner that induces follicle stimulating hormone (FSH) and luteinizing hormone (LH) to be released from the anterior pituitary.
LH acts on theca cells to cause the conversion of cholesterol to
androstenedione by the enzyme desmolase. FSH acts on granulosa cells to
convert androstenedione to estradiol.
Estriol is secreted by the placenta during pregnancy.
Women who are not pregnant produce virtually no estriol. Estriol is the
most predominant estrogen produced by the female during pregnancy. The
production involves the fetal adrenal gland producing dehydroepiandrosterone, which is then converted by the placenta to estriol.
Note: USMLE pearl about these three types of Estrogen Hormones.
1. Estradiol: Is the young female’s hormone of femininity. It’s also called E2.
It’s prododuced by aromatization of Testosterone in the Graafian
follicle (granulosa cell). It’s the most potent estrogen with the
highest effect on receptors.
2. Estrone: Is the estrogen of the menopause. It’s produced by aromatization of androstenedione in peripheral (fatty) tissue. It’s also called E1. It’s less potent than Estradiol.
3. Estriol: Is the placental estrogen and it’s only seen during pregnancy and its high levels reflects fetal well being. It’s also called E3.
It’s the least potent of all estrogens. It originates from the fetal
adrenal gland in the form of DHEA Sulfate and then is finally
transformed to Estriol in the placenta by the sulfatase enzyme.
During pregnancy, estrogens increase contractility of the myometrium by upregulating factors that will aid in delivery of the baby at term.
“Estradiols” Image created by Lecturio
Physiology of progesterone
“Progestins” Image created by Lecturio
Progesterone is produced during the luteal phase of the menstrual cycle. It is produced by the corpus luteum
in the ovary and naturally declines as the menstrual cycle continues.
The corpus luteum can be maintained from pregnancy and continue to
secrete progesterone for the first few weeks. After the loss of the
corpus luteum progesterone levels are maintained by the trophoblast. Finally, the placenta will produce progesterone as the pregnancy progresses.
Progesterone is needed to maintain pregnancy. During conception, progesterone induces the endometrium to prepare for implantation, and if implantation does not occur progesterone levels will drop off and lead to menstruation. Its effect on pregnancy is through stabilization of the myometrium to inhibit contraction; this is a counter action to estrogen’s effect on the myometrium. It also inhibits lactation
until late in the pregnancy and progesterone affects the vaginal
epithelium and cervical mucus, making it thick and impenetrable to
sperm.
Physiology of testosterone
Testosterone is produced by the Leydig cells of the testes. It is also produced from the zona reticularis of the adrenal gland.
The zona reticularis produces DHEA, DHEA sulfate, androstenedione and
11-hydroxyandrostenedione. These are then converted in the peripheral
tissues to the active form testosterone.
There are three mechanisms by which testosterone interacts with cells:
- It can directly bind to an androgen receptor.
- In tissues that contain the enzyme 5-alpha reductase, testosterone can be converted to dihydrotestosterone.
- In tissues that contain aromatase, testosterone can be converted to estradiol.
- In addition, testosterone circulates in three manners:
- A free form
- bound to albumin
- bound to sex hormone binding globulin.
USMLE pearl: A common question involves the 5-alpha
reductase inhibitor finasteride. Dihydrotestosterone plays a big role in
the growth and development of the prostate and hair pattern
development. Due to this, finasteride is used to treat benign prostatic
hyperplasia and male pattern baldness.
Testosterone has a broad range of effects throughout development and in sperm production. It induces differentiation of the genitourinary tract during the 7th week and development for the male genitalia. Leydig cells
are the main producers of testosterone and contribute to the
development of the male gonadal anatomy including the vas deferens,
epididymis and seminal vesicles.
GnRH causes release of LH and FSH from the anterior
pituitary in a pulsatile fashion. LH influences Leydig cells to secrete
testosterone. Combined, they allow for spermatogenesis and maturation
before ejaculation. The pulsatile effect also results in testosterone
influencing development of secondary sexual characteristics. Feedback on
the system comes from elevated testosterone levels, which reduce the
levels of GnRH secretion.
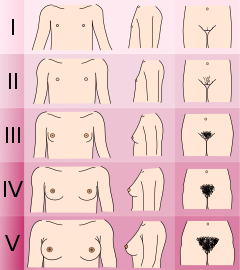
USMLE pearl: It is important to go over Tanner
staging for both males and females. In males, testosterone’s influence
leads to secondary sexual characteristics including muscle and bone
growth, vocal cord thickening and spermatogenesis.
Adapted from text by Lawrence Neinstein, M.D.
Genitals (male)
- Tanner I
testicular volume less than 1.5 ml; small penis of 3 cm or less
(prepubertal) (typically age nine and younger)
- Tanner II
- testicular volume between 1.6 and 6 ml; skin on scrotum thins, reddens and enlarges; penis length unchanged (9–11)
- Tanner III
- testicular volume between 6 and 12 ml; scrotum enlarges further; penis begins to lengthen to about 6 cm (11–12.5)
- Tanner IV
- testicular volume between 12 and 20 ml; scrotum enlarges further and darkens; penis increases in length to 10 cm (12.5–14)
- Tanner V
- testicular volume greater than 20 ml; adult scrotum and penis of 15 cm in length (14+)
Breasts (female)
- Tanner I
- no glandular tissue: areola follows the skin contours of the chest (prepubertal) (typically age 10 and younger)
- Tanner II
- breast bud forms, with small area of surrounding glandular tissue; areola begins to widen (10–11.5)
- Tanner III
- breast begins to become more elevated, and extends beyond the borders of the areola, which continues to widen but remains in contour with surrounding breast (11.5–13)
- Tanner IV
- increased breast size and elevation; areola and papilla form a secondary mound projecting from the contour of the surrounding breast (13–15)
- Tanner V
- breast reaches final adult size; areola returns to contour of the surrounding breast, with a projecting central papilla. (15+)
Pubic hair (both male and female)
- Tanner I
- no pubic hair at all (prepubertal) (typically age 10 and younger)
- Tanner II
- small amount of long, downy hair with slight pigmentation at the base of the penis and scrotum (males) or on the labia majora (females) (10–11.5)
- Tanner III
- hair becomes more coarse and curly, and begins to extend laterally (11.5–13)
- Tanner IV
- adult–like hair quality, extending across pubis but sparing medial thighs (13–15)
- Tanner V
- hair extends to medial surface of the thighs (15+)
Estrogen-related pathophysiology
Loss of estrogen leads to menopause,
which naturally occurs and is defined as 12 months of amenorrhea.
During this time, there is a loss of estradiol production from the
ovaries. The estrogen drop-off is significant with only adipose tissue
still producing estrone. This overall loss of estrogen leads to symptoms
of hot flashes, bloating, mood changes, depression, headache, insomnia. These symptoms often come on before or during the onset of menopause.
Osteoporosis
is commonly seen in conjunction with menopause. It is the result of a
loss of estrogen’s effect on the bone cells – osteoblasts, which
normally inhibit apoptosis,
and on osteoclasts, which normally induce apoptosis. This results in
osteoclasts destroying more bone than osteoblasts can produce. A loss in bone mineral density occurs, which results in an increased risk of fractures of the wrist and hip. The level of loss of bone mineral density is diagnosed with a DEXA scan.
A T-score of -2.5 or lower qualifies as osteoporosis. A T-score of -1.0
to -2.5 signifies osteopenia, meaning below-normal bone density without
full osteoporosis.
Treatments are bisphosphonates such as alendronate (drug names end with -ate).
Calcium and vitamin D
work in conjunction where vitamin D allows for absorption of calcium
from the small intestine and helps with bone mineralization.
Cardiovascular disease also increases at the onset
of menopause. Women lag behind men in average ages for cardiac events by
10 years due to estrogen’s protective effects on cardiac vasculature.
The mechanism is believed to be due to lowering LDL and increasing HDL
levels.
Menopausal treatment is done for symptoms relief.
Estrogen therapy works to help restore the lost estrogens. The therapy
has been used alone and with progesterone/progestins. Estrogen can be
taken orally, transdermally or vaginally. There is an increased risk of endometrial and breast cancer due to estrogen’s trophic effect on the endometrium and breast epithelium.
Testosterone-related pathophysiology
As men age, they gradually decrease the amount of testosterone
they produce at about a rate of 1% from the age of 30 onward. In obese
men, adipose tissue converts testosterone to estradiol by aromatase. The
increased estradiol production causes inhibition of the hypothalamus pituitary axis leading to lower levels of testosterone production.
Much like estrogen, there are three distinct methods to administer exogenous testosterone:
- Oral testosterone agents are the least effective because their absorption undergoes processing by the liver, which results in metabolism of much of the oral form.
- Injections can be done in a depot form with extended discharge, so it can release an average amount per week.
- Topical and transdermal can release low doses on a daily basis.
Exogenous testosterone use has been shown to cause oligospermia and azoospermia due to disruption of the hypothalamus pituitary axis.
In women, excess androgens can lead to physical changes that manifest as acne, hirsutism, virilization and reproductive dysfunction. This is seen in polycystic ovarian syndrome, where excess LH can induce androgen production.
Benign prostatic hyperplasia (BPH) is a very common
condition that occurs as men naturally age. Testosterone acts on the
prostate gland’s growth where it is converted by 5α reductase to
stimulate hyperplasia. This leads to symptoms in men of increased
frequency, urgency, straining, nocturia. BPH is treated with a 5α reductase inhibitor, such as finasteride.
Anabolic steroids are commonly used by weight
lifters to increase their strength in a short period of time. Most often
they will use testosterone and other androgenic compounds which work to
stimulate muscle growth. They can have many adverse effects:
behavioral, endocrine and dermatological.
Antiestrogens
Selective estrogen receptor modulators (SERMs) are used to block estrogen’s function in breast cancer. Many breast cancers
have estrogen receptor α, which can be blocked by the drug tamoxifen.
The selective aspect of SERMs comes from its inhibitory effects on
growth in breast cancer. In the endometrium however, it has a
stimulatory effect causing endometrial hyperplasia,
which puts the woman at risk of endometrial cancer. To mitigate these
effects, progesterone is given to help dampen the hyperplasia.
Aromatase inhibitors can be used to block the production of estrone by aromatase in adipocytes. This can be used to treat gynecomastia.
Antiprogestins
Antiprogestins can be used as forms of birth control.
When combined with a prostaglandin, such as misoprostol, they can be used to induce abortions
as the morning after pill. Mifepristone is used to block progesterone’s
maintenance effect thus stimulating myometrial expulsion of the embryo.
Birth control is usually combined with estrogen and antiprogestin.
The antiprogestin norethindrone inhibits FSH and LH being released from
the anterior pituitary.
Antiandrogens
Spironolactone will block androgen receptors and lower testosterone levels. This can be used to treat the symptoms of polycystic ovarian syndrome in females. Spironolactone also acts as a diuretic acting upon the collecting duct of the nephron.
5α reductase inhibitors, such as finasteride, function to decrease the conversion of testosterone to dihydrotestosterone. Dihydrotestosterone has a stronger effect than testosterone alone, leading to prostatic hyperplasia and male hair pattern. Blocking dihydrotestosterone production mitigates the symptoms of BPH and aids in the treatment of male pattern baldness.

Comentários
Enviar um comentário