Carbonyl Compounds: Nomenclature, Nucleophilic Addition and More
Table of Contents
Image: “Chemistry” by Quinn Dombrowski. License: CC BY-SA 2.0
Carbonyl Compounds
In organic chemistry, a carbonyl compounds are a functional group consisting of a carbon atom with a double bond to an oxygen atom. Many different kinds of carbonyl compounds are present in nature. They all contain an acyl group (R-C=O) with another substituent attached to it. The R part of the structure can be any alkyl, alkenyl or alkynyl group. It can also have other functional groups attached to it.
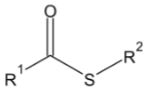
The acyl group is bonded on the other side to either hydrogen, carbon, oxygen, nitrogen, halogen or sulfur. Depending on the different substituents attached to the acyl group, different types of compounds can be formed.
Different types of carbonyl compounds
(Table was prepared by Mark Xavier Bailon)
Carbonyl compounds are generally divided into two groups. One category is composed of the aldehydes and ketones, the other one composed of the carboxylic acids and their derivatives. These two groups generally differ in their kinds of chemistry and reactions.
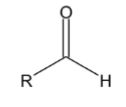
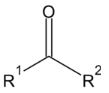
Aldehydes and ketones are functions in the second degree of oxidation. They are considered derivatives of a hydrocarbon by substitution of two hydrogen atoms in the same carbon for one of oxygen, giving rise to an oxo group (= O). If the substitution takes place on a primary carbon, the resulting compound is an aldehyde, and is named with the ending -al. If the substitution takes place on a secondary carbon, it is a ketone, and it is named with the suffix -one.
Aldehydes and ketones, having an H or a C bonded to the acyl group, can’t participate in nucleophilic substitution reactions because these two atoms cannot stabilize a negative charge. Not being able to stabilize negative charges, these substituents cannot participate as a leaving group in nucleophilic substitution reactions.
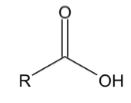
For carboxylic acid and its derivatives, the atom directly attached to the acyl group (oxygen, nitrogen, sulfur, halogen) can stabilize negative charges and, therefore, can participate in nucleophilic substitution reactions.
Carbonyl Structure
The carbonyl group is a functional group composed of carbon and oxygen atoms connected by a double bond. The hybridization of the carbon atom is sp2 and therefore forms three sigma bonds and one pi bond. The oxygen atom, on the other hand, forms one sigma and pi bond to the carbonyl carbon. It also has two non-bonding pairs of electrons.
The difference between the electronegativity values of carbon and oxygen produces a strongly polarized double bond. The oxygen atom is more electronegative and so has a higher tendency to attract electrons, creating a partially negative end. In turn, the carbon atom becomes partially positive because of the pull of electrons toward the oxygen atom.
Nomenclature of Aldehydes and Ketones
In naming aldehydes and ketones, the terminal –e of the corresponding alkane is replaced by –al or –one, respectively. For aldehydes, the parent chain should contain the –CHO group. If the aldehyde functional group is the functional group of highest priority, the carbonyl carbon is assigned as carbon 1. For example, in the case of propane, when two of the hydrogen atoms on the terminal carbon are replaced by an oxygen atom double bonded to the carbon atom, the name of the aldehyde described is propanal.
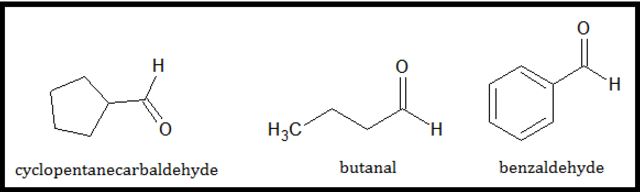
A cyclic aldehyde is a compound where an aldehyde group is directly attached to a ring. In naming these types of compounds, the suffix –carbaldehyde is added to the name of the cyclic compound. For example, a cyclopentane with an aldehyde group attached to it will be named as cyclopentanecarbaldehyde. The figure below shows different aldehyde compounds with their names:
Ketones are named by replacing the terminal –e of the parent hydrocarbon with –one. For compounds containing ketone as the functional group of highest priority, the parent chain is the longest chain containing the ketone functional group. The assignment of a carbon number proceeds in the direction giving the carbonyl carbon the lowest possible number. In the new IUPAC system of naming, the locant number is placed before the suffix.
The parent compound is pentane as it is a 5-carbon alkane. The lowest possible number for the carbonyl carbon is 2 when counting is started from the rightmost carbon atom. In this case, the name of the compound will be pent-2-one.
Nucleophilic Addition
Carbonyl group a) provides a site for the nucleophilic addition and b) increase the acidity of the hydrogen atoms attached to alpha carbon. These two effects are consistent with the structure of the carbonyl groups and are, in fact, due to same ability of oxygen to incorporate a negative charge.
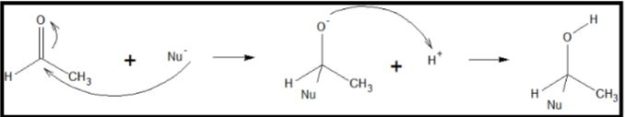
Reactions of aldehydes and ketones are limited only to nucleophilic addition reaction. The attack of the nucleophile is through the electrophilic carbon atom. Carbon atom becomes somewhat electron-deficient because of the pull of oxygen atoms to the electrons. Because of this, the carbonyl carbon becomes highly susceptible to a nucleophilic attack. Since the H and C atom in the substituent is not a good leaving group, only addition of the nucleophile occurs.
A nucleophile is any chemical species that has a high electron density and is attracted to an electrophilic atom in another molecule, such as the low electron density of the carbonyl carbon. In the process of attachment, the double bond between carbon and oxygen is removed, and a localized negative charge is transferred to the oxygen atom forming an alkoxide intermediate. Protonation of the intermediate results to formation of a neutral alcohol addition product. Below is the mechanism of the nucleophilic addition reaction:
Carbohydrates
One important carbonyl compound in nature is the carbohydrate. Carbohydrates are long chain carbon compounds containing a carbonyl group or groups. They are mainly synthesized in nature by a process called photosynthesis. In photosynthesis, plant facilitates the reaction between carbon dioxide and water, in the presence of solar energy, through enzymes present in the plant’s cells, producing carbohydrates and oxygen molecules.
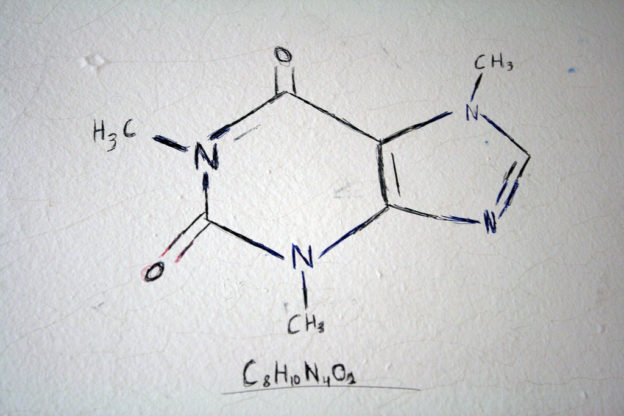
The term carbohydrate was derived from glucose having the molecular formula of C6H12O6. It was originally thought to be a hydrate of carbon. Carbohydrates are referred to as a broad class of polyhydroxylated aldehydes and ketones and are generally called sugars.
The presence of the hydroxyl group in the chain of a glucose molecule enables the compound to also occur in ring forms instead as an open-chain aldehyde. In the case of glucose, the hydroxyl group attached to carbon 5, attacks the carbonyl carbon through the nucleophilic addition mechanism.
Since the attack can occur from the top or from the bottom, the cyclic form of glucose can either be in alpha or beta form. When the –OH group in the anomeric carbon (original carbonyl carbon) is down, the molecule is in alpha form, and when the –OH group is up, it is said to be in its beta form. The specific forms of these sugar molecules are important as it affects its reactivity and its ability to be metabolized by the body.
Carbohydrates provide more than 50% of the energy needed for metabolism, growth, repair, secretion, absorption, excretion and mechanical work.
Metabolism of carbohydrates includes reactions experienced by carbohydrates from food sources or those formed from compounds of other carbohydrates. The oxidation of this type of carbohydrates provides energy as glycogen, the synthesis of non-essential amino acids and in front of the excess of carbohydrate form fatty acids.
Oxidation and Reduction
These reactions occur simultaneously when electrons are transferred. The molecule that is oxidized loses an electron and the molecule that is reduced gains the electron that was lost by the oxidized molecule.
In general, aldehydes are more reactive than ketones because they are less hindered due to the presence of only a H-atom as their substituent instead of an alkyl group. Therefore, aldehydes can also participate in other types of reaction depending on the reaction conditions.
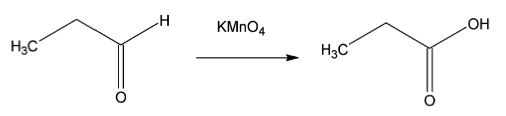
In the presence of strong oxidants like potassium permanganate and potassium dichromate, aldehyde can be oxidized to produce carboxylic acids. For example, when propanal is oxidized by potassium dichromate, propanoic acid is produced.
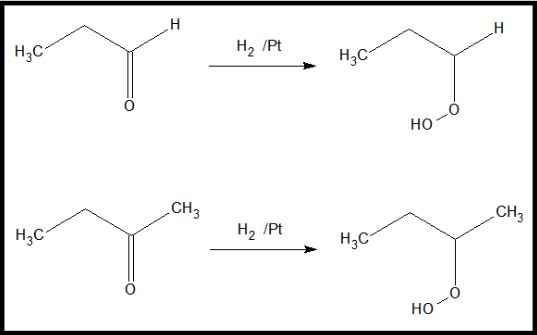
On the other hand, both aldehydes and ketones can undergo reduction reactions. Both can react with reducing agents, such as sodium borohydride or hydrogen gas, in the presence of a Lindlar’s catalyst to produce an alcohol. Aldehydes produce primary alcohols through reduction, while ketones produce secondary alcohols.
Review Questions
The correct answers can be found below the references.
1. What do you call a compound containing an acyl group with only an H atom as a substituent attached to the carbonyl carbon?
- Ketone
- Aldehyde
- Carboxylic acid
- Acid halide
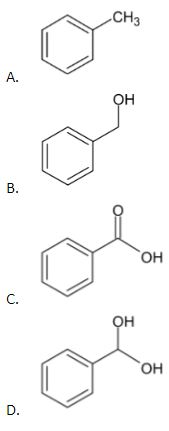
2. When benzaldehyde is oxidized by potassium permanganate, which of the following compounds will be produced?
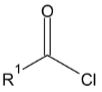
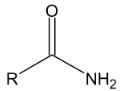
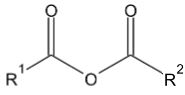
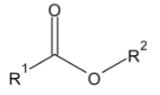
Comentários
Enviar um comentário