Thermodynamics and Thermochemistry: Hess´s Law, Gibb´s Free Energy, and the Coefficient of Thermal Expansion
In this article, you will learn more about thermodynamics and thermochemistry in order to be best prepared for your exam. You will increase your knowledge about Hess´s Law, Gibb´s Free Energy and the coefficient of thermal expansion.
Table of Contents
Are you more of a visual learner? Check out our online video lectures and start your physics course now for free!

Hess’s Law (Hess´s Law of Constant Heat Summation)
Definition
Hess’s Law, also known as Hess’s Law of Constant Heat Summation, stated that no matter how many steps or stages are present in a reaction, the total enthalpy change for the entire reaction is the sum of each individual change. Enthalpy is a thermodynamic measurement assessing the total heat content of a system. It is equivalent to the internal energy of the system, plus the product of the pressure and volume as stated below:
H = U + pV
H = enthalpy of the systemU = internal energy of the system
p = pressure of the system
V = volume of the system
Let us look at a reaction: A → B → C. Here, substance A is undergoing a reaction to become B which then undergoes another reaction to become C. The change in enthalpy of the energy change is denoted by ΔH.
The ΔH for the total reaction is equal to the sum of the ΔH for the first reaction, plus the ΔH for the second reaction.
ΔHA͢͢͢͢͢ to B + ΔHB to C = ΔHA to C
Calculation Using Hess’s Law
Let us see how to understand the actual calculation that takes place in a reaction. Look at the reaction below:
CH4 + 2O2 → CO2 + 2H2O
Here, methane and oxygen react to form carbon dioxide and water. In order for the reaction to occur, the elements need to be re-arranged. This is done by breaking bonds and forming new bonds between elements.CH4 (methane) needs to be broken down into its simple elements: C (carbon) and H (hydrogen). When this occurs, an energy change occurs which is referred to as: (ΔHf)CH4. This is the standard energy of formation referring to standard elements present in nature. For O2 (oxygen), we do not need to break it into individual O elements since, in nature, single O isn’t present. The standard form is O2.
In order to form the products, C and O have to combine to form CO2 (carbon dioxide) and H and O have to combine to form H2O (water). The enthalpies for formation of these two products are: (ΔHf)CO2 and (ΔHf)H2O, respectively.
The total enthalpy of the entire reaction would be as follows:
ΔHreaction = (ΔHf)CO2 + 2(ΔHf)H2O – (ΔHf)CH4
The minus sign for the enthalpy for methane is due to the break-up of the bonds that are taking place.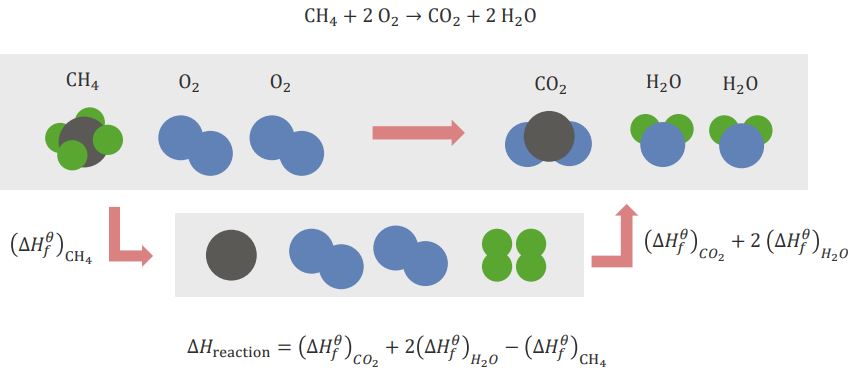
“Hess´s law” Image created by Lecturio
- In determination of the heat of formation
- In determination of the heat of transition
- In determination of the heat of hydration
- To calculate bond energies
Gibb’s Free Energy
Definition
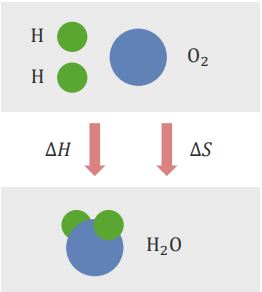
“Gibb´s Free Energy” Image created by Lecturio
Entropy is interpreted as the degree of disorder or randomness that exists in a system. The calculation of the Gibb’s Free Energy (ΔG) can tell if the reaction can occur spontaneously or not.
Enthalpy change (ΔH) and entropy change (ΔS) are competing for events. Enthalpy wants to be minimal in a reaction, whereas entropy wants to be maximal in a reaction.
Also, entropy is associated with temperature since the disorder that exists in a system is dependent on temperature. The higher the temperature, the more the disorder that is present.
The relationship between the Gibb’s Free Energy (ΔG), enthalpy change (ΔH), and entropy change (ΔS) is as follows:
ΔG = ΔH – T ΔS
Where :ΔG = Gibbs free energy
H= enthalpy of the system
TS= random energy
ΔGsystem= -T ΔS total
Note the negative sign since enthalpy and entropy are opposing reaction concepts. Also, temperature (T) is measured in Kelvin (K).
To Be Spontaneous or Not To Be Spontaneous, That is the Question?
The reaction that needs to take place can occur spontaneously (favorably) or non-spontaneously (unfavorably). This is determined by the calculation of the Gibb’s Free Energy (ΔG).
If ΔG < 0, then the reaction is considered to be a spontaneous reaction.
If ΔG > 0, then the reaction is considered to be a non-spontaneous reaction.
Understand that even if the reaction is exothermic (requires no energy input), it might not happen if it lowers the entropy too much. Also, just because something is spontaneous, it does not mean it is quick. Reactions are either spontaneous (favorable) or non-spontaneous (unfavorable). The reaction can also be quick or slow. Both of these concepts are not directly related since they depend on different properties of the reaction.
Coefficient of Thermal Expansion
Definition
The coefficient of thermal expansion describes how the size of different types of matter can be affected (changed) by a change in temperature. Specifically, it measures the fractional change in size per degree change in temperature at a constant pressure. This change can be associated with linear (one-dimensional) change or with volumetric (three-dimensional) change. When an object is heated (implying temperature is increasing), the heated object is larger. Increasing heat will continue causing some expansion to occur based on degree Kelvin change in temperature.Linear Thermal Expansion
An object has a linear dimension which, when heated, undergoes a linear expansion. The linear thermal expansion can only be measured in the solid state and is common in engineering applications. This change in thermal expansion in the linear (one-dimensional) direction is denoted as follows:
Lfinal = Linitial (1 + α ΔT)
Lfinal = final lengthLinitial = initial length
α = coefficient of linear expansion
ΔT = temperature change
The coefficient of linear expansion, α = 1 / temperature; thus, the units for α are 1 / Kelvin.
Volumetric Thermal Expansion
An object has a volumetric dimension which, when heated, undergoes volumetric expansion. The volumetric thermal expansion can be measured for all condensed matter (liquids and solid states). This change in thermal expansion in the volumetric (three-dimensional) direction is denoted as follows:
Vfinal = Vinitial (1 + αv ΔT)
Vfinal = final volumeVinitial = initial volume
αv = coefficient of volumetric expansion
ΔT = temperature change
The coefficient of volumetric expansion, αv = 3 α. This is because the volumetric coefficient is associated with three dimensions, thus, it is equal to three of the coefficient of linear expansions.
Simplifying Thermal Expansion Calculations
The thermal expansion equations for linear expansion and volumetric expansion can be stated in a different manner based on a change in length and a change in volume, respectively.Linear Expansion
Lfinal = Linitial (1 + α ΔT)
Lfinal = Linitial + (Linitial α ΔT)
Lfinal = Linitial + ΔL
So, the change in length, ΔL = Linitial α ΔT.Volumetric Expansion
Vfinal = Vinitial (1 + αv ΔT)
Vfinal = Vinitial + (Linitial αv ΔT)
Vfinal = Vinitial + ΔL
So, the change in volume, ΔV = Vinitial αv ΔT.Application of thermal expansion:
- Thermal expansion is also used in mechanical applications to fit parts over one another.
- For applications using the thermal expansion property, such as bi-metal and mercury thermometer.
Comentários
Enviar um comentário