Steroids and Bile Acid: Lipid Metabolism
Table of Contents
Image: “Skeletal structure of acetyl-CoA with the acetyl group highlighted” by Bryan Derksen (original) and DMacks (color-change). License: Public Domain
Ketone Bodies
Ketone bodies are two chemicals produced when fatty acids are broken down in excess. These compounds are produced in a process called ketogenesis. These three compounds are acetone, acetoacetate and β-hydroxybutyrate. Ketone bodies are synthesized in the liver from acetyl-CoA.
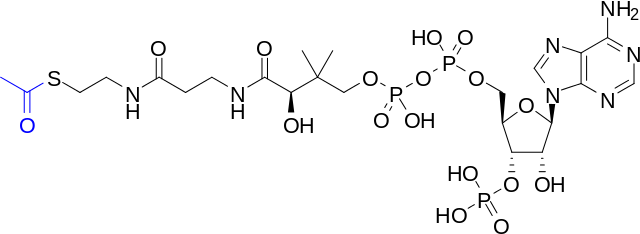
Image: “Skeletal Structure of Acetyl-CoA with the Acetyl Group Highlighted” by Bryan Derksen (original) and DMacks (color-change). License: Public Domain
Synthesis of ketone bodies normally occurs in the body. However, the process of ketone body formation dramatically increases during starvation. A combination of factors may explain this, which includes prolonged low levels of insulin resulting in the increasing in both the release of fatty acid from adipose tissues, and the amount of enzymes required to utilize and synthesize ketone bodies. Also, in the liver, the increase in demand for gluconeogenesis results in a depletion of oxaloacetate, and eventually results to a decreased capacity for the TCA cycle. This results in an increase to the levels of acetyl-CoA, which is the substrate for the production of ketone bodies.
Ketone body synthesis involves the use of a number of enzymes. The first enzyme in the pathway is thiolase. This enzyme catalyzes the synthesis of acetoacetyl-CoA from the condensation of two acetyl-CoA molecules. The next step involves the use of HMG-CoA synthase which adds a third acetyl-CoA molecule to form β-hydroxy-β-methylglutaryl-CoA(HMG-CoA), which is an important intermediate in the synthesis of ketone bodies.
The next step is to release an acetyl-CoA from HMG-CoA to form acetoacetate using HMG-CoA lyase. Another enzyme important in ketone body synthesis is the β-hydroxybutyrate dehydrogenase, which works to reduce acetoacetate to β-hydroxybutyrate.
Prominent Steroid: Cholesterol
In 1926, the Nobel Prize in Chemistry was awarded to Heinrich Wieland for his pioneering work on the structure of cholesterol and bile acids. Several scientists and researchers further investigated on the function of cholesterol in body systems and its synthetic pathway.
Cholesterol is the most prominent member of the steroid family of lipids. It is mostly found in animals and humans as it is the main sterol in these organisms. Only small quantities of this compound are synthesized by plants and fungi. It is an amphipathic lipid – that’s why it is an important structural component of membranes of cellular organelles and the outer layer of the plasma membrane.
All human cells and body fluids, like plasma, contain cholesterol. This may be in the form of free cholesterol or in its storage form which is a cholesteryl ester in which cholesterol is combined with a long chain of fatty acid making the cholesterol molecule more hydrophobic.
Image: “The Structure of Cholesterol” by BorisTM. License: Public Domain
Cholesterol is a sterol because it is a steroid with an alcohol group. It is a derivative of the cyclic hydrocarbon Perhydrocyclopentanophenanthrene. The structure of cholesterol is shown on the right.
It is important to determine the cholesterol level in the blood every now and then because the cholesterol level is an indicator of some heart diseases. The desirable total blood cholesterol level for adults is 200 mg/dL or that’s 5.13 mmol/L. About 50% of adults fall into this category. About 33% of adults are in borderline high risk of coronary heart disease with a total blood cholesterol level of 200–239 mg/dL or 5.13–6.13 mmol/L. Twice the risk of coronary heart disease is 17% of adults with a total blood cholesterol level 240 mg/dL or 6.14 mmol/L and over.
Cholesterol can be synthesized from acetate in all of the tissues that are steroid-producing. Most of the cholesterol synthesis occurs in the liver, the skin, and the intestinal mucosa.
Cholesterol is biosynthesized from the triterpene squalene. The first step in the synthesis is the conversion of squalene to its 2,3-epoxide in the presence of O2, NADH, and enzyme. The next step is an epoxide ring opening reaction that triggers cyclization. In this step, there is a loss of proton that is accompanied by a series of hydride shifts and methyl migrations. The final product for this rearrangement is the triterpene, lanosterol. A series of enzyme-catalyzed reactions then convert lanosterol to cholesterol.
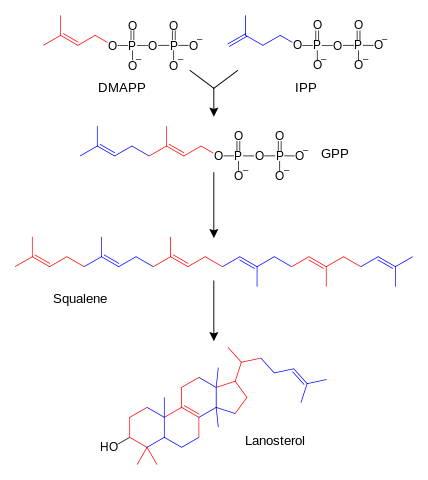
Image: “The Simplified Schematic Diagram of Sterol Biosynthesis. Several Intermediates have been Omitted.” by Fvasconcellos, original by Tim Vickers. License: Public Domain
Receptor-mediated Endocytosis
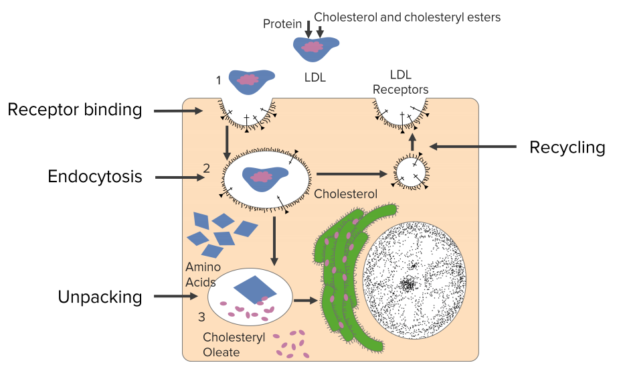
The way that cholesterol actually gets into cells is interesting.The reason that the cholesterol is in the LDLs isn’t to cause heart attacks but rather to provide a delivery mechanism for cells to get cholesterol. Cells have on their surface a receptor that recognizes and binds to LDLs. If the cell is needing cholesterol, it will grab a LDL out of the bloodstream and internalized it in this process seen in the figure.
First of all, a binding process is followed by endocytosis. And this process is called receptor-mediated endocytosis for this purpose. The steps happening inside, there is actually an unpacking of the LDL that occurs and the goodies inside including the cholesterol are used by the cell. There is a recycling that happens where the remnants that aren’t used by the cells are then exported back out of the cell. And in the meantime, the cell has gotten cholesterol that it needs for the purposes of making membranes.
Steroid Metabolism
Steroids are low molecular weight, lipophilic compounds that are derived from cholesterol, and are important in a number of physiological processes. Steroid hormones are mainly produced by the endocrine glands (testis and ovary), and the adrenal gland, and are eventually released into the blood stream.
Steroid plays an important role in co-ordinating physiological and behavioral responses to different biological processes, like reproduction. They also influence the sexual differentiationof the genitalia, as well as the determination of secondary sexual characteristics during sexual maturation and development. They also function in controlling the sexual behaviors of organisms.
The parent compound in all steroid hormones is cholesterol. The first step in the biosynthesis of steroid is the conversion of the 27-carbon skeleton of cholesterol to a 21-carbon pregnenolone. The pregnenolone is then converted to either progesterone, which proceeds to the androgen/estrogen pathways, or to 17α-hydroxpregnenolone, which provides another route for the production of androgen and estrogens.
The biosynthesis of steroid is made possible by a number of enzymes belonging to four main classes. The first class of enzymes is called desmolases. Desmolases or lyases are enzymes which work in removing parts of the original cholesterol side-chain. This enzyme requires the presence of cytochrome P-450, molecular oxygen and the reduced form of nicotinamide dinucleotide phosphate (NADPH) as a co-factor. They are generally located in the mitochondria and are involved in an electron transport chain.
Another important group of enzymes is the hydroxylases, which are membrane-bound protein enzymes which work to remove hydroxyl groups from the parent compound. Another group of an enzyme is the hydroxysteroid dehydrogenases, which are oxidoreductases that catalyze reversible redox reactions. The last important group of the enzyme is the aromatases, which convert the A-ring in the cholesterol structure to a phenolic group in a process known as aromatization.
Bile Acids – End Product of Cholesterol Utilization
Bile acids are the end products of cholesterol utilization. Synthesis of bile acids is a result of the cholesterol catabolism in mammals. The liver is the only organ where complete biosynthesis of bile acids occurs. The process of bile acid production is one of the predominant mechanisms to excrete excess cholesterol. However, this process is still insufficient to remove the excess dietary intake of cholesterol.
The major pathway for bile acid synthesis is initiated via hydroxylation of cholesterol at the 7 position in the presence of the enzyme cholesterol 7α-hydroxylase. Another pathway for bile acid synthesis is through the hydroxylation of cholesterol at the 27 position by the mitochondrial enzyme sterol 27-hydroxylase.
Review Questions
The correct answers can be found below the references.
1. Which of the following enzymes is responsible for converting the A ring in the cholesterol into a phenolic group?
- Aromatase
- Dehydrogenase
- Desmolases
- Hydroxylases
2. What is the parent compound in the biosynthesis of steroids?
- Bile acid
- Cholesterol
- Ketone bodies
- Squalene

Wow, excellent post. I'd like to draft like this too - taking time and extremely hard work to make a great article. This post has inspired me to write some posts that I am going to write soon on Buy C4-Pharma Combo 450
ResponderEliminar