Hemoglobin and Movement of Oxygen
Table of Contents
Hemoglobin
Hemoglobin (commonly abbreviated as Hb) is a metalloprotein inside red blood cells that can carry oxygen from the lungs to the tissues and organs in the body. When it goes back to the lungs, it carries the carbon dioxide for eventual release to the body. This protein is essential as the oxygen it transports is needed by the body for metabolism.
In mammals alone, this metalloprotein comprises 96% of the red blood cells dry content. Its oxygen binding capacity is 1.34 mL of Oxygen (O2) per gram. Mammalian hemoglobin can bind up to 4 oxygen molecules. Aside from transporting oxygen, it is also used to transport carbon dioxide and other gases, like nitric oxide, which is a regulatory molecule for the body.
Hemoglobin Structure
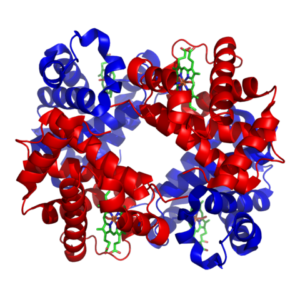
Image: “Haemoglobin” by Richard Wheeler (Zephyris) 2007. License: CC BY-SA 3.0
Hemoglobin of vertebrates is a globular protein with aquaternary structure that consists of four polypeptide chains, two alpha, and two beta chains. Each of these chains has oxygen-binding heme or iron protoporphyrin. Depending on the species, slight differences in the number of amino acids comprising the chains may be observed. For human hemoglobin, there is a slight difference in the number of amino acids present in the alpha and beta chain. For the alpha chain, there are 141 amino acids, while for the beta chain, there are 146 amino acids.
Each of the alpha and beta chains is folded to contain characteristic protein secondary structures. They have a number of alternating alpha helixes and beta sheets in their structure. The tertiary structure of each chain is very similar to that of myoglobin. The confirmation of the alpha and beta chains only differs by an additional helix in the beta chain. The different chains are connected together by non-covalent interactions. These include hydrogen, ionic and hydrophobic bonds between the alpha and beta subunits, as well as between the two dimers.
Each of the alpha and beta subunits contains the heme group in the center. The heme group needs to be in a hydrophobic environment and so it is buried deep in the hydrophobic pockets of the E and F helices of the chains. Some amino acids like histidine and phenylalanine in the structure around the heme group further increase the hydrophobicity of the environment around the metalloporphyrin.
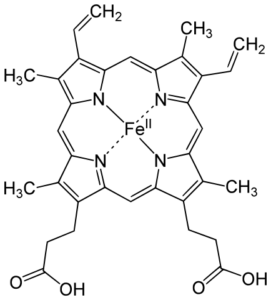
Image: “Heme b Group” by Yikrazuul – Own Work. License: Public Domain
The heme group is a complex of protoporphyrin IX and a ferrous iron (Fe2+). The ferrous ion is present in the middle, connected via a co-ordinate covalent bond to four nitrogen atoms in the porphyrin ring. It is also connected to a histidine residue in the globin chains. Because of this, the heme group can carry only one oxygen. Since there are four chains present in each hemoglobin molecule, four oxygen atoms can be carried.
Oxygen Binding
Hemoglobin exists in two forms depending if an oxygen is attached to it or not. The first form is called the T-form, or the taut (tense) form. This is the form of the hemoglobin when it is deoxygenated. In this form, the movement of the dimers is constrained. The other form is called R-form, or relaxed form. This is the form of the hemoglobin molecule when it is oxygenated. The dimers in this form have more freedom of movement.
Different factors affect oxygen binding to the metalloporphyrin groups in the hemoglobin molecule. These include pH of the environment, partial carbon dioxide pressure (pCO2), temperature, 2,3-bisphosphoglycerate availability and initial oxygen binding.
The presence of carbon dioxide also improves the stability of deoxyhemoglobin. It stabilizes deoxyhemoglobin by binding to the terminal amino group, forming a negatively-charged carbamate group. The negative charges form salt bridges to positively charged amino groups and side chains. In this way, the T-form of hemoglobin is stabilized and the system then favors the release of oxygen.
The pH of the environment also affects the binding of oxygen to the hemoglobin molecule. Decreasing pH of the environment helps stabilize the deoxygenated form of hemoglobin, thus decreasing its affinity for oxygen. An acidic environment promotes the formation of a bond between histidine 146 and the lysine residue in the alpha subunit of the alpha-beta dimer. The hemoglobin molecule changes conformation in such a way that it prevents rebinding of the oxygen molecule after its release.
In the human red blood cells, 2,3-bisphosphoglycerate (2,3-BPG) exist normally at 5 mmol/L concentration. This is almost the same value to the intracellular concentration of hemoglobin. In the deoxygenated form, hemoglobin molecule has the 2,3-BPG in the spaces between the H-helices of the beta chain. This molecule fits well in the central cavity. The bond between hemoglobin and the BPG needs to be broken to convert from the T-form to the R-form. After oxygenation, the 2,3-BPG is expelled from the cavity.
Temperature also affects the oxygen-hemoglobin binding. In cases of extreme hypothermia, the affinity of hemoglobin for oxygen increases. Compared when the body is at 37 deg Centigrade, hemoglobin has 22 times greater affinity for oxygen at 0 deg Centigrade.
It was found that the resulting graph for oxygen dissociation is S-shaped, or sigmoidal. This means that, as the partial pressure of oxygen increases, the hemoglobin molecule becomes increasingly saturated with oxygen. This is due to the changes in the shape and conformation of hemoglobin as oxygen binds. The pattern shows that the second and third oxygen molecule binds easier, compared to the binding of the first and last oxygen molecule.
Bohr Effect
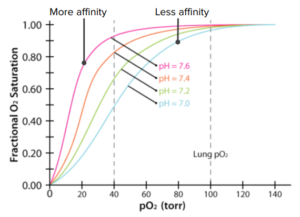 Protons can bind to hemoglobin.
Protons can bind to hemoglobin.- Protons change hemoglobin´s shape.
- Reshaped hemoglobin loses oxygen.
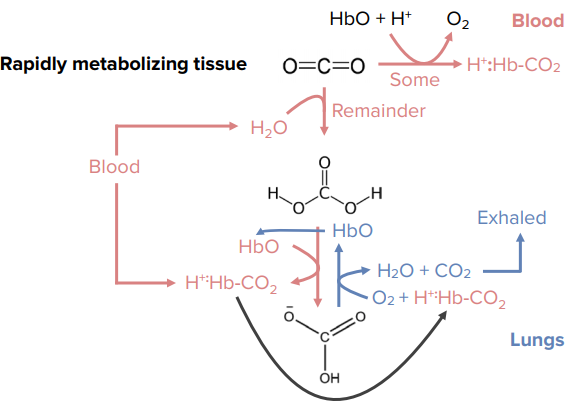
- Rapidly metabolizing tissues release protons.
- Rapidly metabolizing tissues get more oxygen from hemoglobin.
Bohr effect & CO2
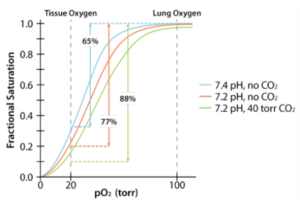 Acid favors release of CO2 from hemoglobin.
Acid favors release of CO2 from hemoglobin.- CO2 favors release of O2 from hemoglobin.
- Acid and CO2 are released by rapidly metabolizing tissues.
2,3 BPG
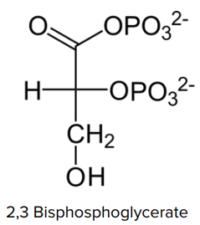 Byproduct of glycolysis.
Byproduct of glycolysis.- Exercising muscle cells rapid use glycolysis.
- Exercising muscle cells produce acid, CO2, and 2,2 BPG.
- Binds in hole of donut.
- Locks hemoglobin in T-state.
2,3 BPG and oxygen binding
- Rapidly metabolizing cells produce acid.
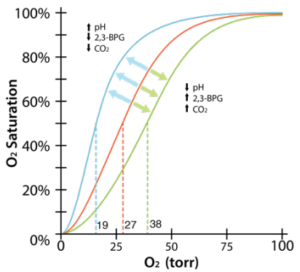 Rapidly metabolizing cells release CO2.
Rapidly metabolizing cells release CO2.- Rapidly metabolizing cells release 2,3 BPG.
- All favor O2 release from hemoglobin, so rapidly metabolizing cells get more O2.
2,3 BPG and smoking
- 2,3 BPG big concern for smokers.
- Blood of smokers has high levels of 2,3 BPG.
- Hemoglobin gets locked in T-state in passage through lungs.
- Oxygen carrying capacity of blood reduced.
- Carbon monoxide levels are also higher in smokers.
Movement of CO2
- An additional histidine is present at the heme iron site.
- Reduces affinity to CO, but does not eliminate it.
- Carbon monoxide in cigarette smoke.
- Note that CO2 does not bind to heme, nor do protons.
Fetal Hemoglobin
- The body makes different globins over time.
- Most variations centered on birth.
- Fetal hemoglobin mostly α2γ2.
- Fetal hemoglobin can´t bind to 2,3 BPG.
- Mostly remains in R-state.

Comentários
Enviar um comentário