Acid Base Balance and its Disturbances: Acidosis and Alkalosis
Table of Contents
- Definition and Introduction
- Volatile and Non-Volatile Acids
- Classification of Acid-Base Balance Disorders
- Metabolic Acidosis
- Metabolic Alkalosis
- Respiratory Acidosis
- Respiratory Alkalosis
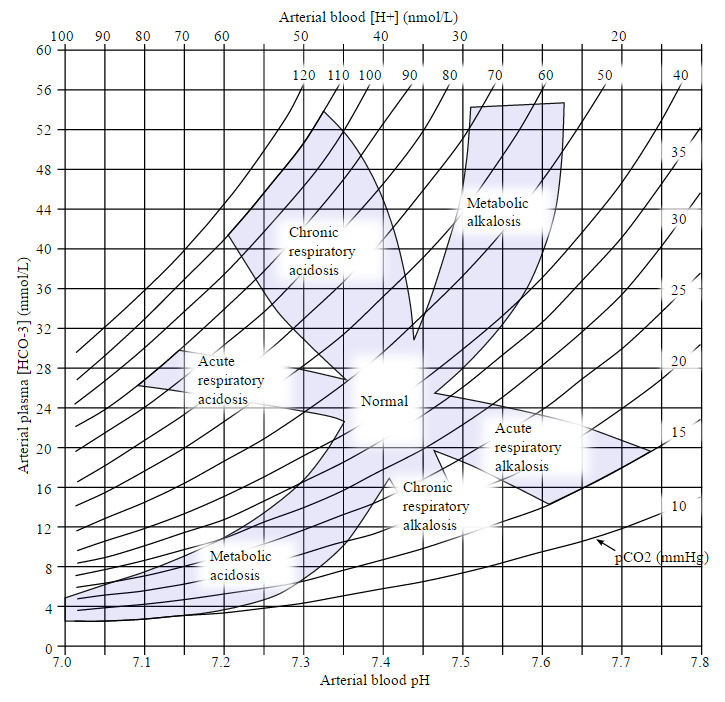
- Diagnosis of Acid-Base Disturbances
- Renal Tubular Acidosis
- Type 1—Distal Renal Tubular Acidosis (RTA)
- Type 2—Proximal Renal Tubular Acidosis (RTA)
- Type 4—Renal Tubular Acidosis (RTA)
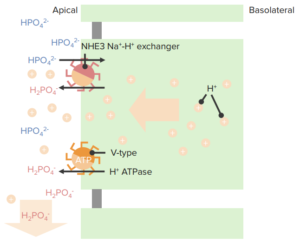
- Different Types of Renal Tubular Acidosis
- References
Image: “PB030008.jpg.” by Matt Ritchie. License: < "https://creativecommons.org/licenses/by/2.0/">CC BY 2.0
Definition and Introduction
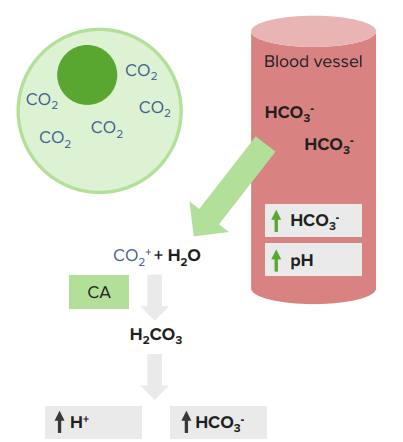
Acid-Base disorders are characterized by abnormal levels of pH, partial pressure of carbon dioxide (Pco2) and bicarbonate (HCO3−) in the blood.
- State of blood in which pH is < 7.35 is Acidemia.
- State of blood with serum pH > 7.45 is called alkalemia.
Acidosis defines all processes in which acid accumulation or alkali loss leads to a reduction in pH level. Alkalosis is characterized by all processes resulting in excess production of alkali or excess acid loss.
Physiologic compensatory mechanisms are activated in the body; change in pH levels depends on the degree of these compensatory mechanisms.
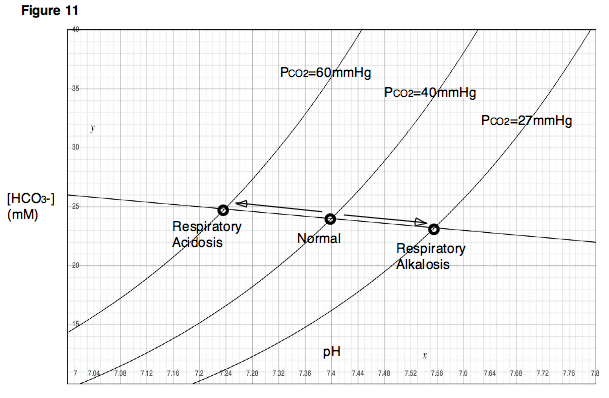
Image: “An acid base nomogram for human plasma, showing the effects on the plasma pH when carbonic acid (partial pressure of carbondioxide) or bicarbonate occur in excess or are deficient in the plasma” by Huckfinne – Own work. License: Public Domain
Volatile and Non-Volatile Acids
Volatile Acids
- CO2
Non-volatile acids
- Sulfur-containing a.a. (e.g., cysteine)
- Phosphorus-containing compounds
- Cationic a.a. (e.g., lysine)
- Uric acid
- Lactic acid
- Keto-acids
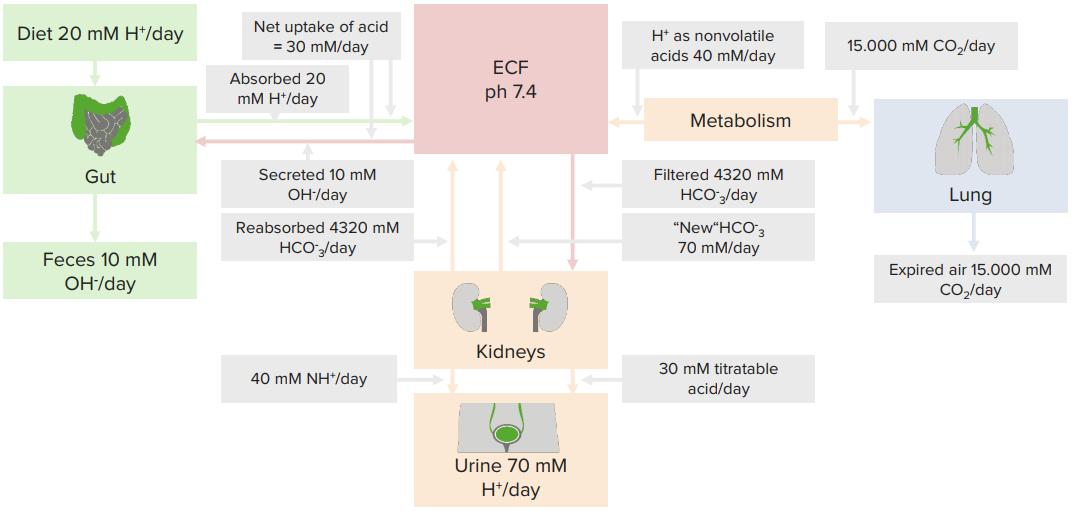
Typical daily acid handling
Titratable acids
 Phosphate (pK = 6.8)
Phosphate (pK = 6.8)- Urate (pK = 5.8)
- Creatinine (pK = 5.0)
- Lactate (pK = 3.9)*
- Pyruvate (pK = 2.5)*
* minor roles
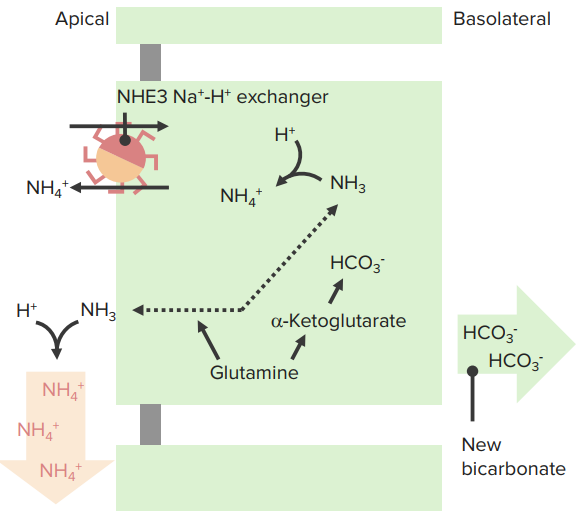
NH3/ NH4+
- NH3 is membrane permeable
- “New” HCO3- ∼ 60 % in PT
Classification of Acid-Base Balance Disorders
Clinically, the primary causes of acid-base imbalance responsible for changes in pH, (and thus changes in serum HCO3− or in Pco2 ) can be:
- Metabolic
- Respiratory
Metabolic acidosis and metabolic alkalosis are caused by an imbalance in the production of acids or bases and their excretion by the kidneys.
Respiratory acidosis and respiratory alkalosis are caused primarily by changes in carbon dioxide exhalation due to lung or breathing disorders.
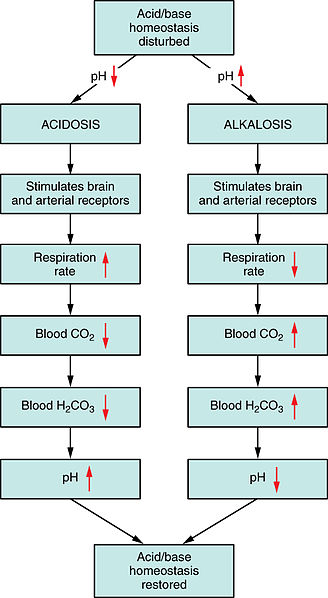
Image: “Respiratory Regulation of Blood.” by Anatomy & Physiology, Connexions. License: CC BY-SA 3.0
Metabolic Acidosis
If serum HCO3− is less than 24 mEq/L, it is termed as metabolic acidosis. The body compensates by hyperventilation, i.e. the rate of breathing increases and CO2 is lost from the body. The body tries to maintain pH through compensatory mechanisms. It implies a high or normal anion gap where anions may or may not be present.
Causes
Causes of metabolic acidosis are an increase in acid production, excess acid intake, reduced excretion of acid by kidneys or loss of negatively charged bicarbonates by GI and kidneys.
Before discussing the causes of acidosis, we need to learn about the anion gap.
Anion gap: It is the sum of the concentration of all the major cations (sodium + potassium ion concentration) minus the sum of the concentration of all the anions (chloride + bicarbonate ions). Positive ions are more in concentration than the negative ions by roughly 14 mmol/L. The normal anion gap is 8—16 mmol/L without potassium in the equation, and 10—20 mmol/L with potassium.
The anion gap exists because there are more unmeasured anions (phosphates, lactate, and sulfate) than cations (includes potassium, calcium and magnesium).
The anion gap exists because there are more unmeasured anions (mostly albumin, but others include lactate and sulfate) than cations (includes calcium and magnesium).
Metabolic acidosis is divided into increased and normal anion gap.
- Increased anion gap:
- Lactic acidosis: shock, infection, hypoxia
- Renal failure
- Raised ketones (diabetes mellitus (DKA), alcohol)
- Drugs or toxins: salicylates, metformin, ethylene glycol, methanol, cyanide
- Normal anion gap (due to loss of bicarbonate or ingestion of hydrogen ions):
- Renal tubular acidosis
- Addison’s disease
- Pancreatic fistulae
- Drugs or toxins: acetazolamide, ammonium chloride
DKA is a complication of uncontrolled type I diabetes mellitus in which surplus ketone bodies are produced as a result of gluconeogenesis.
Lactic acidosis is due to surplus lactic acid production in the body as a result of:
- Prolonged exercise and lack of oxygen
- Low blood sugar, or hypoglycemia
- Alcohol use
- Liver failure
- Severe dehydration or shock
Hyperchloremic acidosis occurs after the loss of bicarbonate through the stools in severe watery diarrhea.
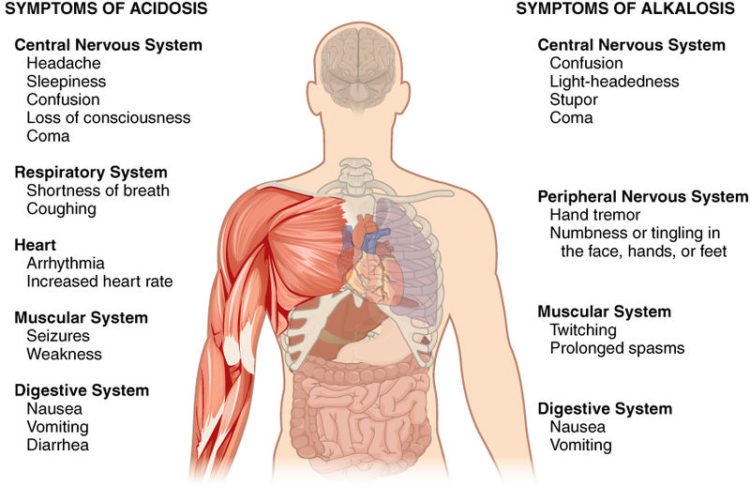
Image: “Symptoms of Acidosis and Alkalosis.” by Anatomy & Physiology, Connexions. License: CC BY-SA 3.0
Clinical Symptoms
In mild metabolic acidosis, people may have no symptoms but usually experience:
- Nausea
- Vomiting
- Fatigue
Kussmaul breathing is a typical breathing pattern in metabolic acidosis, i.e. deeper and slightly faster (the body tries to correct acidosis by removing CO2). In extreme cases, cardiac issues may develop and the blood pressure falls, leading to shock, coma and even death.
Metabolic Alkalosis
If the arterial blood gas and electrolyte show alkalemia and serum HCO3− is more than 24 mEq/L, it will be labeled as metabolic alkalosis.
Causes
- Vomiting
- Hypokalemia—e.g., diuretics. See also the separate article on hypokalemic alkalosis
- Excessive alkali drugs, such as for acid dyspepsia
- Burns
A major cause of metabolic alkalosis is the loss of stomach acid after persistent vomiting.
The body compensates by reducing the respiratory rate and retaining CO2, which tries to maintain body pH.
The symptoms of metabolic alkalosis are a headache, lethargy, and tetany.
Respiratory Acidosis
Respiratory acidosis is a state of the body in which partial pressure of the CO2 is more than 40 mm Hg (hypercapnia), along with acidic arterial pH.
Causes
Acute Onset Acidosis:
- Depression of the central respiratory center by cerebrovascular disease or drugs (coma)
- Inability to ventilate adequately due to neuromuscular disease e.g., myasthenia gravis, amyotrophic lateral sclerosis, Guillain-Barré syndrome (GBS), muscular dystrophy
- Airway obstruction related to asthma or exacerbation of chronic obstructive pulmonary disease (COPD)
Acute acidosis is a worse form of respiratory acidosis that implies symptoms like a headache, confusion, stupor, drowsiness with the signs like tremors, myoclonic jerks, and asterixis.
Chronic Onset Acidosis:
Chronic respiratory acidosis can occur secondary to COPD, obesity hypoventilation syndrome (Pickwickian syndrome), neuromuscular disorders and restrictive ventilatory defects, such as in interstitial fibrosis or thoracic deformities. Chronic acidosis is asymptomatic.
Respiratory Alkalosis
When Pco2 falls below 40 mm Hg (hypocapnia) along with alkalemia in the arterial blood, it is called respiratory alkalosis.
pH level may be near normal or high. Respiratory alkalosis may be acute or chronic in onset. The symptoms of acute respiratory alkalosis are light-headedness, confusion, cramps, paresthesias and syncope with the signs of hyperpnea or tachypnea and spasms. Chronic one remains asymptomatic.
Causes
Increase in minute ventilation (persistent hyperventilation) due to any cause like anxiety, stroke, meningitis, altitude, and pregnancy can lead to respiratory alkalosis.
Diagnosis of Acid-Base Disturbances
For the diagnosis of acidosis or alkalosis, the following investigations are needed:
- Arterial blood Gases—ABG with pH, Pco2, Po2
- Serum electrolytes
- Anion gap calculated
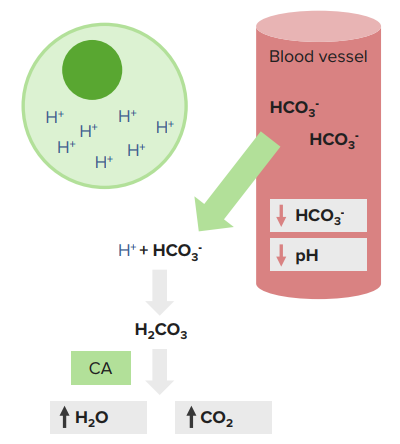
The ABG directly measures arterial pH and Pco2. In case of CVS failure, venous blood gasses VBG may more accurately reflect conditions at the tissue level and may be a more useful guide to bicarbonate administration and adequacy of ventilation. Bicarbonates levels on ABG are directly measured and calculated using the Henderson-Hasselbalch equation.
Renal Tubular Acidosis
Renal tubular acidosis (RTA) is a type of acidosis in which an electrolyte disturbance in the body leads to a chronic metabolic acidosis with a normal anion gap. RTA can occur after abnormal renal H+ ion excretion (type 1 RTA) or impaired bicarbonate resorption (type 2 RTA), or abnormal aldosterone production or response (type 4 RTA). Type 3 RTA is a very rare condition, so it is not discussed. RTA, if not managed properly, can complicate chronic renal failure CRF.
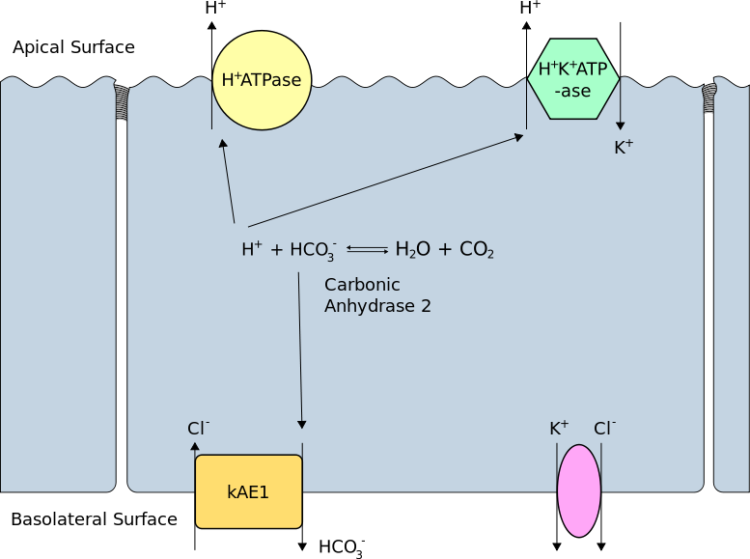
Type 1—Distal Renal Tubular Acidosis (RTA)
H+ ion secretion into the Distal Tubule by the alpha-intercalated cells of the medullary collecting duct is impaired in type I RTA, resulting in a high urine pH (> 5.5) and metabolic acidosis. In distal RTA, serum HCO3 < 15 mEq/L along with hypokalemia, hypercalciuria, and decreased citrate excretion. Nephrocalcinosis and nephrolithiasis are possible complications and presentation of many patients of distal RTA.
Causes
It can be primary or secondary; familial cases manifest in childhood and are autosomal dominant (AD).
Secondary causes include:
- Autoimmune disease with hypergammaglobulinemia, particularly Sjögren syndrome or RA
- Kidney transplantation
- Nephrocalcinosis
- Medullary sponge kidney
- Chronic obstructive uropathy
- Drugs (mainly amphotericin B, ifosfamide, and lithium)
- Cirrhosis
- Sickle cell anemia
Type 2—Proximal Renal Tubular Acidosis (RTA)
The main etiology behind this is the inability to reabsorb bicarbonate in the Proximal Tubules with urine pH > 7 if plasma bicarbonate concentration is normal, and urine pH < 5.5 if plasma bicarbonate concentration is already depleted due to ongoing losses. The generalized inadequate function of proximal tubules contributes to this syndrome. These patients can have an increased urinary excretion of glucose, uric acid, phosphate, amino acids, citrate, calcium, potassium, and proteins as well.
Patients develop osteomalacia or osteopenia (rickets in children). Hypercalciuria, hyperphosphaturia, disturbed Vitamin D metabolism and secondary hyperparathyroidism are the major complications.
Causes
- Fanconi syndrome
- Light chain nephropathy due to multiple myeloma
- Various drug exposures (usually acetazolamide, sulfonamides, ifosfamide, outdated tetracycline or streptozocin)
Type 4—Renal Tubular Acidosis (RTA)
Aldosterone deficiency or loss of sensitivity of the collecting ducts to aldosterone is the main cause of type 4 RTA. Reduced potassium excretion leads to hyperkalemia and reduced acid excretion leading to mild metabolic acidosis with a normal anion gap (i.e., a hyperchloremic acidosis) because of the renal ability to compensate. This is the most common type of RTA. Urine pH is usually similar to serum pH. It is < 5.5 when there is serum acidosis.
It’s secondary to impairment in the renin-aldosterone-renal tubule axis in:
Other factors that can contribute to type 4 RTA include the following:
- ACE inhibitor use
- Aldosterone synthase type I or II deficiency
- Angiotensin II receptor blocker use
- Chronic kidney disease, usually due to diabetic nephropathy or chronic interstitial nephritis
- Congenital adrenal hyperplasia, particularly 21-hydroxylase deficiency
- HIV nephropathy (due, possibly in part, to infection with Mycobacterium avium complex or cytomegalovirus)
- Interstitial renal damage (e.g., due to SLE, obstructive uropathy or sickle cell disease)
- Potassium-sparing diuretics (e.g, amiloride, eplerenone, spironolactone, triamterene)
- Obstructive uropathy
- Primary adrenal insufficiency
- Aldosterone deficiency is the most frequently seen form of hyperkalemic metabolic acidosis in adults
- Pseudohypoaldosteronism (type I or II)
- Volume expansion (e.g., in acute glomerulonephritis or chronic kidney disease)
great article, which is dangerous respiratory or metabolic?
ResponderEliminarAcidosis and Alkalosis