Arterial Blood Gas (ABG) Analysis
Table of Contents
Image: “Cobas b 121 (Roche Diagnostics) – measurement chamber (detail). From the left: Reference electrode Sample’s conductivity electrode Na+ electrode Cl- electrode pH (glass) electrode Sample’s conductivity electrode Ca2+ electrode K+ electrode pO2 (Clark) electrode pCO2) (Sveringhaus) electrode Hemoglobin/Hematocrit chamber. Emergency Laboratory, Hospital de Niños, Córdoba, Arg.” by J3D3 – Own work. License: CC BY-SA 3.0
Overview of ABG Analysis
Analysis of arterial blood gases (ABG) is an essential investigation in the management of critically ill patients as it provides important information about alveolar ventilation, oxygenation, and acid-base balance.
Measurement of ABG includes measurement of three parameters, i.e., pH, PaCO2, and PaO2; however, measurement of bicarbonate (HCO3–) and electrolytes is almost always done simultaneously to complete the whole picture of acid-base & electrolyte balance/imbalance.
Important values
pH
- Normal blood pH: 7.36–7.44
- Acidemia: pH < 7.35
- Alkalemia: pH > 7.45
PaCO2 & PaC02
- Normal PaO2: 80–100 mm Hg
- Normal PaCO2: 36–44 mm Hg
- Respiratory acidosis: Acidemia with PaCO2 > 44 mm Hg
- Respiratory alkalosis: Alkalemia with PaCO2 < 36 mm Hg
HCO3–
- Normal HCO3-: 20–28 mEq/L
- Metabolic acidosis: Acidemia with HCO3– < 20 mEq/L
- Metabolic alkalosis: Alkalemia with HCO3– > 28 mEq/L
- Normal base excess/deficit: 0 ± 2 mEq/L
Anion Gap
- Normal Anion Gap (AG): 12 mEq/L
- Anion Gap Metabolic Acidosis (AGMA): Metabolic acidosis with AG > 12
- Non-Anion Gap Metabolic Acidosis (NAGMA): Metabolic acidosis with normal AG
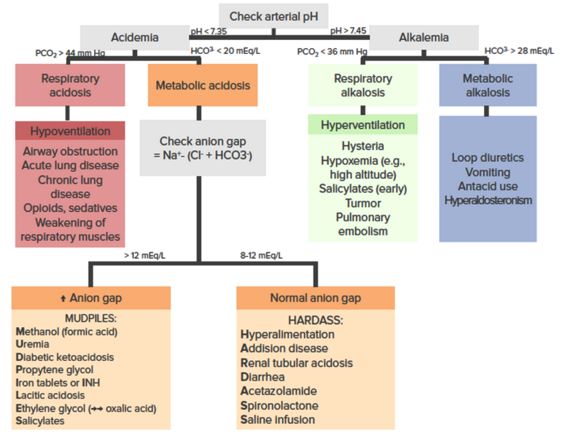
“Acidosis and Alkalosis” by Kapil Pal
Basic Physiological Concepts
PaCO2 is the best parameter that reflects alveolar ventilation; high (> 44 mm Hg) and low (< 36 mm Hg) PaCO2 levels suggest alveolar hypoventilation and alveolar hyperventilation respectively.
The status of oxygenation is affected mainly by PaO2, FiO2, and hemoglobin content along with its affinity and saturation with oxygen. The presence of hypoxemia (PaO2 < 80 mm Hg at sea level while breathing room air) does not necessarily mean the presence of hypoxia.
Blood pH is maintained in the narrow range (7.36–7.44) by lungs, kidneys and blood buffers. In general, the blood buffer system (H2CO3/HCO3–) acts within a fraction of seconds, the respiratory system takes about 1–15 minutes, and kidneys may take many minutes to many days to adjust H+ ions concentration.
PaCO2 is mainly controlled by lungs and is a marker of respiratory acid-base disturbance. HCO3- is mainly controlled by kidneys and blood buffers, but it does not always purely indicate metabolic acid-base disturbance as it is affected by hydrolysis effect.
Plasma bicarbonate can be calculated using the Henderson-Hasselbalch equation; while standard bicarbonate is plasma bicarbonate obtained after blood has been equilibrated at 37°C with a PaCO2 of 40 mm Hg.
Henderson-Hasselbalch equation: pH = 6.1 + log10 ([HCO3-]/0.03 x PaCO2)
The difference between the measured bicarbonate and the calculated bicarbonate is known as base excess (if positive value) or base deficit (if negative value).
In acidemia, an excess of H+ ions enter intracellular space, hence, K+ ions move out from intracellular space into the plasma to maintain electroneutrality. Thus, acidemia is associated with hyperkalemia.
In distal renal tubular cells, K+ or H+ ions are exchanged for Na+ ions. In alkalemia, as H+ ions are retained, K+ ions are exchanged for Na+ ions, leading to hypokalemia.
Compensation: In response to primary acid-base disturbance, the respiratory system or kidneys attempt to maintain normal pH by the compensatory response. Compensation “moves the pH towards normal”, but compensation can never “overshoot” the normal pH.
Respiratory compensation for a primary metabolic derangement is immediate and usually improves over the course of hours, while renal compensation for a primary respiratory derangement is gradual and improves over days as it takes time for the kidneys to generate/eliminate HCO3.
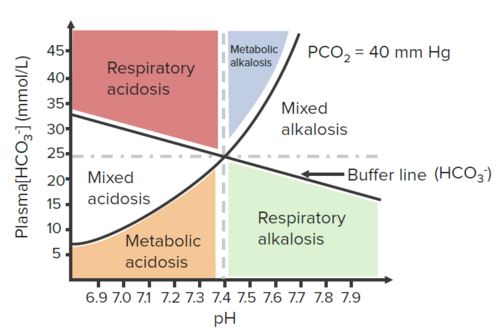
“Davenport (Acid/Base) Diagram” by Kapil Pal
Anion Gap (AG)
Calculation of anion gap is very important for metabolic acidosis. In electroneutrality:
Unmeasured anions + Cl– + HCO3– = Unmeasured cations + Na+
Unmeasured anions are usually proteins, SO4, PO4, organic anions, etc. and unmeasured cations are K+, Ca++, Mg++, etc. Therefore:
Anion Gap (AG) = Na+ – (Cl– + HCO3–) = Unmeasured anions – Unmeasured cations
An elevated anion gap refers to an increase in unmeasured anions. Normal AG: 12 mEq/L
Respiratory Acidosis
Key features: pH < 7.35 with PaCO2 > 44 mm Hg
Causes: Basically, causes of respiratory acidosis are those that cause CO2 retention or hypoventilation.
- Upper airway disorders such as obstruction, laryngospasm, obstructive sleep apnea
- Lower airway disorders such as COPD, asthma
- Thoracic cage abnormalities restricting respiratory movements such as flail chest, kyphoscoliosis
- Neuromuscular disorders affecting ventilation such as AIDP
- CNS depression due to CNS disorders, opioids, sedatives, etc.
- Respiratory muscle fatigue following any prolonged/severe respiratory disease
Respiratory Alkalosis
Key features: pH > 7.45 with PaCO2 < 36 mm Hg
Causes: Basically, causes of respiratory alkalosis are those that cause CO2 washout or hyperventilation.
- Hyperventilation due to pain, anxiety, hysteria, CNS tumor/disorder, pulmonary embolism, etc.
- Hypoxemia due to high altitude or any other cause
- Early salicylate poisoning
Metabolic Acidosis
Key features: pH < 7.35 with HCO3– < 20 mEq/L
Metabolic acidosis is further sub-classified on the basis of anion gap.
Anion Gap (AG) = Na+ – (Cl– + HCO3-)/p>
Causes of high Anion Gap Metabolic Acidosis (AGMA) (AG > 12 mEq/L):
- Methanol (formic acid) poisoning
- Uremia
- Diabetic ketoacidosis, Drugs
- Propylene glycol poisoning
- Iron poisoning, Isoniazid
- Lactic acidosis
- Ethylene glycol (→ oxalic acid) poisoning, Ethanol poisoning (Alcoholic ketoacidosis)
- Salicylate poisoning
⇒ Mnemonic: MUDPILES
OR
- Lactic acidosis
- Uremia
- Salicylate poisoning
- Iron poisoning, Isoniazid
- Diabetic ketoacidosis, Drugs
- Alcohol poisoning (methanol, ethanol, ethylene glycol, propylene glycol)
⇒ Mnemonic: LUSID Alcohol
Causes of Normal Anion Gap Metabolic Acidosis (NAGMA):
- Hyperalimentation (TPN)
- Aldosterone related (Addison disease, spironolactone, RTA type IV)
- Acetazolamide
- Renal tubular acidosis
- Diarrhea, ureteral Diversion (ileal loop), after DKA treatment
- Fluids (after volume resuscitation with low HCO3– fluids such as normal saline)
⇒ Mnemonic: HAARD Fluids
Metabolic Alkalosis
Key features: pH > 7.45 with HCO3– > 28 mEq/L
Metabolic alkalosis is further sub-classified into saline-responsive (generally low urine Cl–) and saline-resistant (generally high urine Cl–) metabolic alkalosis. Causes of saline-responsive metabolic alkalosis:
- Gastrointestinal losses (emesis, nasogastric tube aspiration, villous adenoma, etc)
- Diuretics
- Post-hypercapnia
⇒ Mnemonic: GDP
Causes of saline-resistant metabolic alkalosis:
- Licorice ingestion
- Mineralocorticoid excess (hyperaldosteronism, Cushing’s syndrome, exogenous administration)
- Bartter’s syndrome
- Exogenous Alkali load
- Gitelman’s syndrome
⇒ Mnemonic: LiMi BAG
Stepwise Approach to Interpret ABG Analysis
Step 1: Is there acidemia or alkalemia?
- If pH < 7.35 → Acidemia
- If pH > 7.45 → Alkalemia
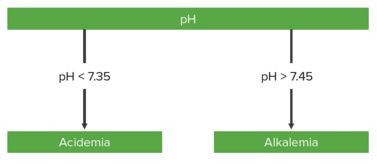
“Step 1” by Kapil Pal
Step 2: Use PCO2 to determine the primary derangement
In acidemia:
- If PCO2 > 40 mm Hg → Respiratory acidosis
- If PCO2 < 40 mm Hg → Metabolic acidosis
In alkalemia:
- If PCO2 > 40 mm Hg → Metabolic alkalosis
- If PCO2 < 40 mm Hg → Respiratory alkalosis
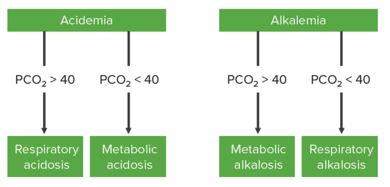
“Step 2” by Kapil Pal
Step 3: Determine if compensation is adequate
For primary metabolic derangement, respiratory compensation is usually immediate and improves over the course of hours. For primary respiratory derangement, renal compensation is usually gradual and improves over days. It also depends on whether the condition is acute or chronic.
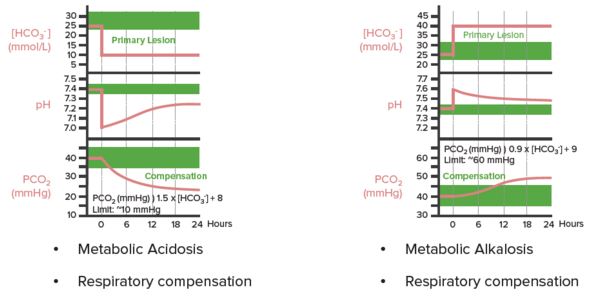
“Arterial Blood Gases” by Kapil Pal
In respiratory acidosis, for every 10 mm Hg increase in PaCO2:
- In acute condition: pH decreases by 0.08 and HCO3– increases by 1
- In chronic condition: pH decreases by 0.03 and HCO3– increases by 4
In respiratory alkalosis, for every 10 mm Hg decrease in PaCO2:
- In acute condition: pH increases by 0.08 and HCO3– decreases by 2
- In chronic condition: pH increases by 0.03 and HCO3– decreases by 4
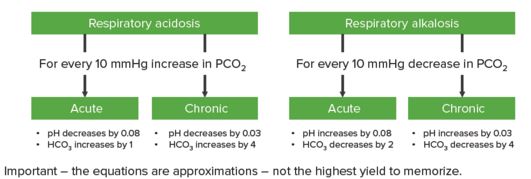
“Step 3” by Kapil Pal
Step 3a: If the expected value for the level of compensation does not correlate with the measured value (pH or HCO3-), then there is likely a mixed disorder present
In metabolic derangements:
- If PCO2 is higher than expected → Concomitant respiratory acidosis
- If PCO2 is lower than expected → Concomitant respiratory alkalosis
In respiratory derangements:
- If pH is higher than expected → Concomitant metabolic alkalosis
- If pH is lower than expected → Concomitant metabolic acidosis
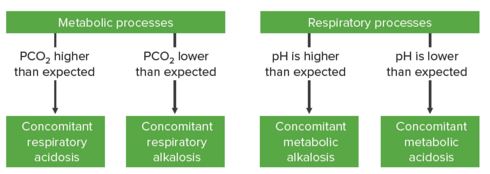
“Step 3a” by Kapil Pal
Step 4: For all metabolic acidosis, calculate the Anion Gap
Anion gap = Na+ – (Cl- + HCO3-) (Normal: 12 mEq/L)
AG should be corrected for hypoalbuminemia: For every 1 mg/dL below 4 → subtract 3 to the “normal” anion gap.
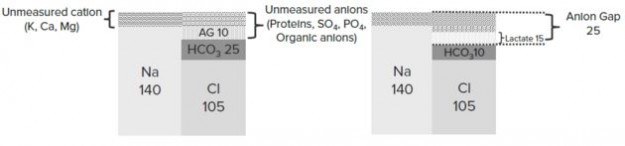
“Step 4” by Kapil Pal
For elevated anion gap metabolic acidosis (AGMA):
Important work up for AGMA includes urine ketones, renal function tests, serum lactate, toxin screen and osmolar(OG). (See the causes.)
- OG = Measured osmolality – Calculated osmolality
- Calculated osmolality = 2(Na+) + Glucose/18 + BUN/2.8
- If OG > 10 → suggests the possibility of ingestion as a cause of AGMA
Another important consideration is to calculate ΔAG (change in AG) and ΔHCO3– (change in bicarbonate).
ΔAG = Patient’s AG – Normal AG
- If ΔAG = ΔHCO3– → pure AGMA
- If Δ AG > Δ HCO3– → concomitant metabolic alkalosis
- If Δ AG < Δ HCO3– → concomitant non-anion gap metabolic acidosis or chronic respiratory acidosis
Example 1: HCO3– = 16, AG = 20
Unmeasured anions Δ AG = 20 – 12 = 8 (normal AG = 12)
Δ HCO3– = 24 – 16 = 8 (normal HCO3– = 24)
Thus, ΔHCO3– = Δ AG = 8 → pure AGMA
Example 2: HCO3– = 10, AG = 20
Unmeasured anions Δ AG = 20 – 12 = 8
ΔHCO3– = 24 – 10 = 14
Thus, Δ HCO< 3- ≠ Δ AG
8 mEq of change in HCO3– is accounted for by AG acidosis; additional (14 – 8=) 6 mEq of change in HCO3– must have been caused by non-AG acidosis → combined AG and non-AG acidosis.
For normal anion gap metabolic acidosis (NAGMA):
Check Urine Anion Gap: UNa+ + UK+ – UCl–
- If urine AG is positive, it implies HCO3– loss by the kidneys as in renal tubular acidosis.
- If urine AG is negative, it implies HCO3– loss from an external source as in gastrointestinal losses.
Step 5: Review differential diagnosis
Refer to causes of acid-base disorders described above and correlate them with the conclusion before making a final diagnosis.
Review Questions
The correct answers can be found below the references.
1. Which of the following is not associated with high anion gap metabolic acidosis?
- Salicylate poisoning
- Iron poisoning
- Acetazolamide poisoning
- Ethanol poisoning
- Isoniazid poisoning
2. Which of the following conditions is associated with saline-responsive metabolic alkalosis?
- Post-hypercapnia
- Licorice ingestion
- Cushing’s syndrome
- Exogenous alkali administration
- Gitelman’s syndrome
3. In a patient with metabolic acidosis, anion gap is 22 mEq/L and serum HCO3- is 16 mEq/L. What is the acid-based imbalance?
- Pure anion gap metabolic acidosis
- Anion gap metabolic acidosis with concomitant non-anion gap metabolic acidosis
- Anion gap metabolic acidosis with concomitant respiratory acidosis
- Anion gap metabolic acidosis with concomitant metabolic alkalosis
- Anion gap metabolic acidosis with concomitant non-anion gap metabolic acidosis and respiratory acidosis
Comentários
Enviar um comentário