Lipid Control: HMG-CoA Reductase Inhibitors
Table of Contents
Image: “Structure of the HMGCR protein” by Emw. License: CC BY-SA 3.0
Overview: Cholesterol and Lipoproteins
Cholesterol is part of the lipids family. They serve many functions in the body. Aside from cellular structure, it also plays a significant role in hormone production and lipid transport, among its many other functions. Cholesterol also plays a major part in the structure of lipoproteins, the primary transport mechanism of lipids in the plasma.
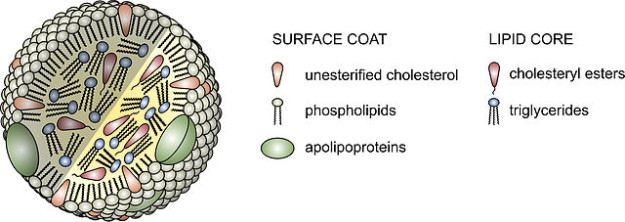
Image: “Lipoprotein particles are composed of a lipid core containing cholesteryl esters and triglycerides, and a surface coat of phospholipids, unesterified cholesterol and apolipoproteins.” by AntiSense. License: CC BY-SA 3.0
A lipoprotein is made up of 2 essential parts: its hydrophobic core and hydrophilic shell. This structure helps it to transport non-polar triglycerides and other lipids along a polar pathway created by the plasma, lymph and interstitial fluid. Its core is made up of cholesteryl esters and triglycerides, while the shell is composed of unesterified cholesterol, phospholipids and apoproteins.
Lipoproteins are classified according to their densities which are dictated mostly by the amount of lipids contained in their core. Since lipids are known to be less dense than water (or protein), lipoproteins, which contain the most lipids in their core, are said to be low-density.
Chylomicrons
Being the group with the most lipids, chylomicrons are said to be the largest among the cholesterols. It stands out among the rest of the groups as the only one which carries exogenous or dietary fat from the gut.
Very-Low-Density Lipoproteins (VLDL)
Next to the chylomicrons in terms of size and density are VLDL. These lipoproteins carry triglycerides from the liver to the peripheries where it is hydrolysed into fatty acids and other forms of cholesterols. The former of these products are used for adipose storage or as fuel for muscles that use ketones for energy such as the heart and skeletal muscles.
Low-Density Lipoproteins (LDL)
LDL is one of the lipoproteins produced when VLDL are deprived of their triglyceride content. They carry the majority of the cholesterol in the body. Their degradation and excretion ultimately allow for bile acid formation to occur. They are known as the ‘bad cholesterol’.
Lp(a) Lipoprotein
This lipoprotein is somewhat similar to a potential thrombolytic compound called plasminogen (active form: plasmin) in structure, and has been found in most atherosclerotic plaques. It is formed by when an (a) protein covalently bonds with an LDL.
High-Density Lipoprotein (HDL)
Like the rest of the lipoproteins, the shell of these so-called ‘good cholesterol’ is secreted by the liver. However, the thing that sets this group apart is that it derives its hydrophobic contents from the triglycerides and cholesterols found lying around in the peripheries. It helps out in the eventual metabolism and excretion of this lipids.
Lipid Control
Metabolism of Cholesterol
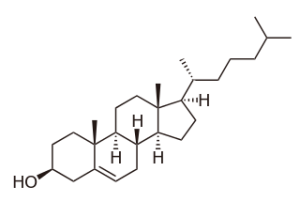
Image: “Structure of Cholesterol” by BorisTM. License: Public Domain
We derive almost half of our cholesterol source from our diet; the rest of it is thanks to our body. As long as we have nucleated cells, we will be able to synthesize our own cholesterol from products of the metabolism of other macronutrients. 10% of our ability to do this is shouldered by our liver and intestines.
It all starts with acetyl coenzyme a or acetyl-CoA, a product of the catabolism of glucose, amino acids and fatty acids. It undergoes a series of reactions in order for it to be ultimately converted into cholesterol.
First off, acetyl-CoA is converted into mevalonate. This involves the creation of, 3-hydroxy-3-methylglutaryl-CoA or HMG-CoA, an intermediate which has to be produced inside the mitochondria. Before this occurs, 2 molecules of acetyl CoA have to be condensed to form acetoacetyl-CoA. Another molecule of acetyl-CoA comes into the picture to condense with this product, forming HMG-CoA which is then reduced to mevalonate with the help of the rate-limiting enzyme HMG-CoA reductase.
Once mevalonate is produced, it is then phosphorylated and decarboxylated to form an isoprenoid unit. Each unit is made up of isopentyl diphosphate. This reaction is repeated until 6 isoprenoid units are produced. These are then isomerized into dimethylallyl diphosphateand condensed with another isoprenoid unit to form geranyl diphosphate. Yet again, another isoprenoid unit condenses with this to form farnesyl diphosphate. It takes two of these compounds to form squalene by condensation.
Since cholesterol needs a steroid nucleus for its chemical structure, the cell has to convert squalene into something that would resemble one, such as lanosterol. This happens as squalene undergoes a series of reactions with the enzymes squalene epoxidase and oxidosqualene-lanosterol cyclase.
Cholesterol is finally created in the membranes of endoplasmic reticulum once the side chains and nucleus of lanosterol are changed by a series of reactions.
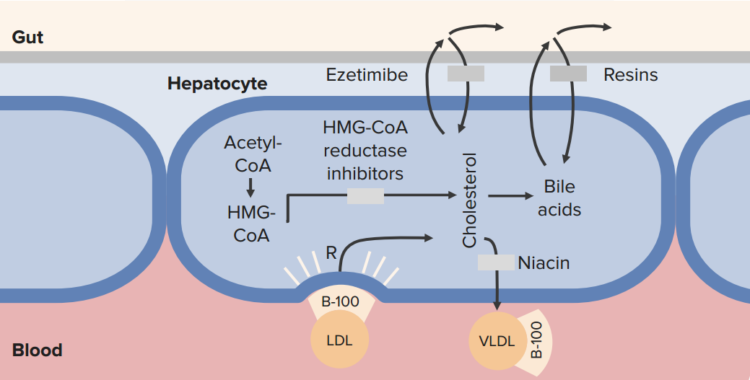
Cholesterol Transport Inhibitors: Ezetimibe
- Prodrug, undergoes glucuronidation in liver
- Inhibits cholesterol transporter: prevents absorption of dietary cholesterol and phytosterol, reactive increase in LDL receptors
Uses:
- Treatment of hypercholesterolemia (20 % reduction as monotherapy, 25–30 % add-on reduction with statin)
- Treatment of phytosterolemia
Toxicity
- Well tolerated
- May increase hepatotoxicity
Sold as:
- Ezetrol®
- Vytorin®
Disorders Affecting Cholesterol Metabolism
Screening
Screening for patients who might be at risk for developing lipoprotein disorders are usually part of routine examinations. Therefore, encountering these problems, along with its associated complications, are somewhat common in practice. This is done by extracting blood samples from patients after doing a 12-hour overnight fast.
Dyslipidemias
In general, problems in lipid metabolism are categorized into two. If they are not associated with other conditions such as any concurrent disease, poor diet or long-term drug therapy, they are thought to be a primary dyslipidemia. Single-gene mutations are usually implicated in this classification. Examples are outlined in the table:
(Table adapted from Harrison’s Principles of Internal Medicine, 19th ed. by Fauci AS, et al.)
Problems in lipid metabolism can also exist as a manifestation of other disorders or conditions in the body. In this case, they are classified as secondary dyslipidemias and may be caused by the following conditions:
- High-carbohydrate diet
- Excessive alcohol intake
- Obesity and insulin resistance
- Excessive glucocorticoid levels
Treatment
The approach to patients with problems in cholesterol metabolism are ideally individualized per patient. It usually is geared towards the prevention of complications, such as the development of cardiovascular diseases. One of the first steps in addressing this goal is the assessment of the patient’s cardiovascular risks.
Lifestyle modification is usually a part of the management of patients with hypercholesterolemia. For obese patients, weight loss to achieve ideal body weight is highly recommended. Nutrition counseling is also advocated in order for patients to maintain proper nutrition, without significantly increasing lipid levels in the body.
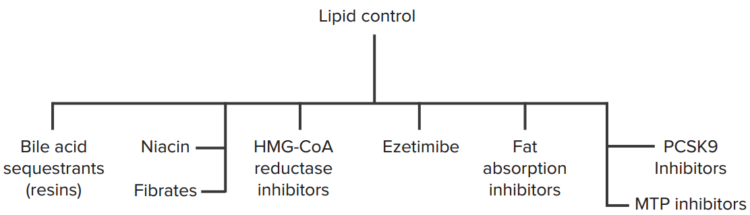
Along with lifestyle changes, the addition of pharmacologic therapy to reduce plasma levels of cholesterol may also be instigated. Medications typically given to patients with hypercholesterolemia may be classified into any of the following groups:
- HMG-CoA reductase inhibitors
- Cholesterol absorption inhibitors
- Bile acid sequestrants
Among these medications, the HMG-CoA reductase inhibitors, otherwise known as statins, are frequently the first medications to be prescribed.
Bile acid sequestrants (resins)
Cholestyramine, colestipol, colesevelam
92 % of bile acids are reabsorbed normally
- Resins prevent reabsorption
- Liver needs to make more bile acids
- Liver uses up cholesterol to do so
- Sucks up more LDL from the blood to use as fuel
Effects:
- Mild lDL reduction
- May increase TG and VLDL in FH and FTG
HMG-CoA Reductase Inhibitors
- Lovastatin
- Atorvastatin
- Fluvastatin
- Pravastatin
- Simvastatin
- Rosuvastatin
- Pitavastatin
Mechanism of action

Image: “HMG-CoA Reductase Pathway (Cholesterol)” by BorisTM. License: CC BY-SA 3.0
These medications work by acting as analogs to 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) and are very effective in reducing LDL. From the name itself, HMG-CoA reductase inhibitors, or statins, interfere with the rate-limiting first step of cholesterol synthesis – the conversion of acetyl acetate into mevalonate. This drug also increases the ability of LDL receptors in the hepatocytes to bind with LDL, thereby increasing their degradation and excretion from the circulation. It acts primarily on the liver, and is linked to significant decreases in LDL, moderate decreases in triglycerides and slight increases in HDL.
It is also part of most health care institutions to include HMG-CoA reductase inhibitors in the treatment plan of patients who have had acute coronary syndromes regardless of their cholesterol levels. This is because HMG-CoA reductase inhibitors are also proven to reduce vascular damages brought about by oxidation. They also stabilize atherosclerotic lesions, preventing further development of complications from atherosclerotic plaques.
- Block a step in the conversion of HMG-CoA to cholesterol
- Competitive inhibitor of the HMG-CoA reductase enzyme
- Most of the effects due to LDL expression
Secondary effects:
- May prevent bone loss
- Anti-inflammatory effects
- Anti-atherosclerotic effects (anti-inflammatory)
- May be protective against contrast nephropathy
Indications
Statins can be prescribed as monotherapy, or in combination with other lipid-reducing medications such as bile acid sequestrants, niacin or ezetimibe. They are usually prescribed for patients suffering from hyperlipidemia (hypercholesterolemia). They are also given to patients with coronary heart disease and atherosclerosis as prophylaxis for myocardial infarction and stroke.
Precautions
Since these medications interfere with the synthesis of cholesterol needed for fetal development, they are not recommended for use during pregnancy. Caution is also observed in giving these medications to children with familial lipid metabolic disorders.
Side Effects
Since statins primarily exert their effects on hepatocytes, most of the side effects seen with these medications are associated with altered liver functioning; one of which is the elevation of serum aminotransferase activity. However, this does not usually warrant discontinuation as long as no other debilitating signs and symptoms of liver toxicity appear. The use of statins among patients with concurrent liver diseases or a history of alcohol abuse may be accompanied by anorexia, malaise and an undesirable rapid drop of LDL levels in the circulation. For this reason, statins are given in reduced dosages for these patients. Other side effects that may be seen are the following:
- Increase in fasting plasma glucose
- Slight increases in creatinine kinase (skeletal muscle weakness)
- Myoglobinuria
- Myopathy
Toxicity
- Mild elevation of CK
- Mild transaminitis
- Potentially fatal reaction in patients on statins plus fibrates (i.e. cerivastatin and gemfibrozil)
- May also react similarly with Niacin
- Metabolized by CYP450 3A4 (grapefruit juice — furanocoumarins; bitter oranges — citrus aurantium; or marmalade)
Drug and Food Interactions
Lovastatin, simvastatin and atorvastatin are specifically catabolized in the liver through the CYP3A4 enzyme system. Food and medications that are catabolized through this system somewhat displace these particular statins, thereby increasing their levels in the blood. Some medications that cause this include:
- Macrolides
- Cyclosporine
- Ketoconazole
- Some HIV protease inhibitors
- Tacrolimus
- Nefazodone
- Fibrates
- Paroxetine
- Venlafaxine
- Grapefruit juice (> 1L/day)
In contrast, CYP3A4 statins are inhibited by medications that increase the expression of CYP3A4 enzymes:
- Phenytoin
- Griseofulvin
- Barbiturates
- Rifampin
- Thiazolidinediones
Fluvastatin, rosuvastatin and pitavastatin are metabolized using the CYP2C9 enzyme system. Their levels are increased in the plasma by medications that inhibit the expression of CYP2C9 enzymes, including:
- Ketoconazole
- Metronidazole
- Sulfinpyrazone
- Amiodarone
- Cimetidine
Red yeast rice, a fermented product used as a natural food coloring and fermentation agent in Asian cuisine, is known to have statin activity and therefore should not be eaten when taking statins.
Fibrates
Peroxisome-activated receptor α (PPAR-α)
- Increased gene expression of ApoA-I, -II, and ApoC-III which increases HDL levels
- Increased gene expression for lipoprotein lipase to help clear TG at endothelium, and increase FFA oxidation in liver
Uses:
- Treat hypertriglyceridemia
- Toxicity
- May increase LDL
- Rash, increased WBC, potentiate anticoagulants
- Risk of myopathy
Important Points
- Statin effect is mostly due to increased LDL receptor expression and NOT the reduction of internal cholesterol biosynthesis
- Normal LDL receptors are required for statins to work
- Homozygous familial hypercholesterolemia is a disease of dysfunctional LDL receptors, statins don´t work well in these patients!
Review Question
The correct answer can be found below the references.
A newly admitted patient with a chief complaint of chest pains is undergoing a series of routine and specific laboratory tests. While waiting for the results to come in, which among the following manifestations would warrant you to delay the administration of statins to the patient?
- A blood pressure reading of 165/100 mmHg
- Yellowish discoloration of the sclerae, skin and mucous membranes
- Headache and dizziness
- None of the above
Comentários
Enviar um comentário