Biochemistry for Physicians: Cholesterol Metabolism
Table of Contents
Image: “Cartoon representation of the molecular structure of protein registered with 1ldl code.” by Jawahar Swaminathan and MSD staff at the European Bioinformatics Institute. License: Public Domain
Cholesterol Structure & Function
Cholesterol is an amphiphillic molecule, consisting of four nonpolar hydrocarbon rings (A-D); a branched nonpolar hydrocarbon tail attached to carbon 17, and a polar alcohol group on carbon 3. Cholesterol’s polar structure allows it to fit inside the phospholipid bilayer and serve as a membrane fluidity buffer.
Cholesteryl esters are cholesterol molecules that have a fatty acid group attached to the alcohol group at position 3 in the “A-ring”, and are more hydrophobic than unesterified cholesterol. Molecules of this type are too hydrophobic to serve as a membrane fluidity buffer and are associated with lipoproteins; or as components of bile.
Cholesterol Biosynthesis
Cholesterol is primarily synthesized in
- liver
- Intestines
- Adrenal cortex
- Reproductive tissues
The carbon skeleton that makes up its structure comes from Acetyl-CoA, whether derived from glucose or fatty acid oxidation. Synthesis takes place in the cytosol, and because this is an anabolic metabolic pathway, NADPH serves as the provider of reducing equivalents.
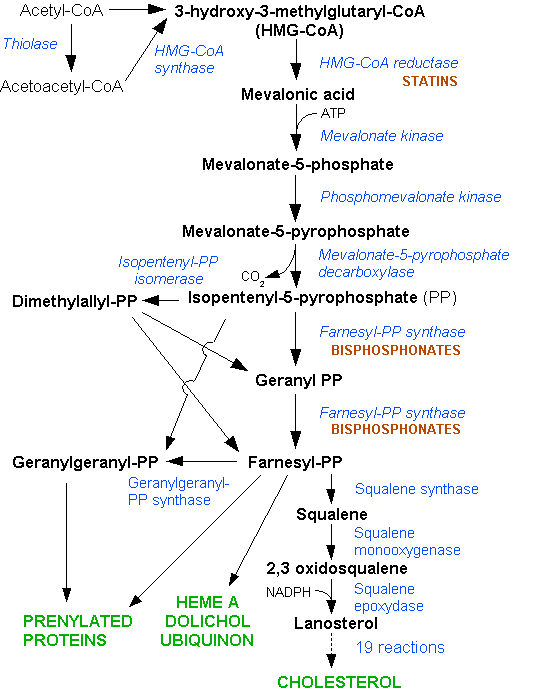
Image: “HMG-CoA reductase pathway” by Dodo. License: CC BY-SA 3.0
The first phase of cholesterol biosynthesis is the formation of mevalonate, which takes place in a sequence of three reactions, by Thiolase (ACAT), HMG-CoA synthase (HMGCS), and HMG-CoA reductase (HMGCR), respectively. In these reactions CoA groups are being cleaved off with only the reductase step using reducing equivalents, in the form of 2 NADPH spent to produce mevalonate. HMG-CoA Reductase is the rate-limiting step in the pathway, and also serves as the key regulatory enzyme for the synthesis of cholesterol. Later we will discuss how a class of drugs called Statins inhibits cholesterol synthesis by attenuating HMGCR.
Note: Note that HMG-CoA has an alternative metabolic fate: When blood glucose levels are low, Acetyl-CoA produced from β-Oxidation of fatty acids is used to generate HMG-CoA, which can be converted to Ketone bodies by the actions of HMG-CoA Lyase (HMGCL), β-hydroxybuterate dehydrogenase, and Acetoacetate decarboxylase (AAD).
Mevalonate Kinase (MVK) converts Mevalonate to mevalonate-5-phosphate by hydrolyzing ATP; and then mevalonate-5-phosphate is phosphorylated a second time by Phosphomevalonate kinase (PMVK) to produce mevalonate-5-pyrophosphate in a reaction that also uses ATP.
The Starter unit of cholesterol synthesis, Δ3-Isopentenyl-PP, is formed by the ATP-dependent action of diphosphomevalonate decarboxylase (MVD), which can readily be converted by Isopentenyl-P2 Δ-isomerase (IDI-1) to the repeating unit of cholesterol synthesis, dimethylallyl pyrophosphate. The starter unit and repeat unit are joined together by dimethylallyl transferase (DMAT) to form geranyl-pyrophosphate, which undergoes conversion to farnesyl-pyrophosphate by geranyl transferase.
This linkage of start and repeat units occurs by “head-to-tail” nucleophilic substitution and the subsequent formed geranyl-pyrophosphate serves as the primer for an analogous condensation yielding farnesyl-pyrophosphate.
Squalene is formed from the “head-to-head” condensation of 2 farnesyl-pyrophosphate molecules by Squalene synthase (SQS), in a reaction that requires NADPH as a cofactor. Eukaryotic cells and prokaryotes differ in their sterol synthesis at this juncture, as our cells use require the presence of oxygen (and NADPH) to fuel the reaction (squalene monooxygenase) that forms squalene-2, 3-epoxide. Prokaryotes anaerobically produce a similar compound to cholesterol, called hopanoids.
Lanosterol synthase produces lanosterol; the first compound on the way to cholesterol that has four closed defined rings, and from this point a sequence of 19 reactions proceeds with the removal of three methyl groups in the formation of zymosterol. Zymosterol’s double bonds are rearranged, and eventually, one is removed, depending on the tissue in which the synthesis takes place. The conversion of lanosterol to zymosterol takes place by enzymes that require eleven NADPH, and cholesterol’s synthesis from zymosterol requires an addition two NADPH in the rearrangement/removal of double bonds.
An important conversion of cholesterol that takes place is esterification with long chain fatty acids (e.g.; palmitic acid) in the endoplasmic reticulum, which blocks the polar head group of cholesterol, and thus its inclusion as a membrane fluidity buffer.
Cholesterol degradation leads to bile acids, which occurs in the liver and proceeds with the excretion of bile into the small intestine. Bile acid amides form conjugates with taurine or glycine, which dissociate completely at physiological pH levels due to their low pKa values. Therefore bile serves as good anionic detergents for the body, and form cylindrical micelles. They act as emulsifiers of lipids in the intestine, and their structures allow for them to be readily resorbed by the intestinal mucosa.
Regulation of Cholesterol Synthesis
The formation of mevalonate by HMG-CoA Reductase is strictly regulated, as it is the key reaction in the pathway leading to cholesterol. Glucagon, (and thus cAMP) induces HMG-CoA Reductase Kinase to phosphorylate HMG-CoA, thereby inhibiting the enzyme. HMG-CoA will be shuttled to the mitochondria for the production of ketone bodies instead. When there is adequate glucose intake, insulin induces HMG-CoA Reductase Phosphatase to dephosphorylate the enzyme, which allows for the production of mevalonate, and eventually cholesterol.
The transcription of HMG-CoA reductase is also under tight regulation so that the synthesis of cholesterol only takes place when the cell has adequate precursors to do so. When the cell needs to produce cholesterol, and there are plenty of precursors, Sterol regulatory element-binding protein (SREBP) is released from the ER and translocates to the nucleus where it binds the sterol regulatory element (SRE). This increases the rate of HMG-CoA reductase transcription.
Conversely, when there are high levels of cholesterol, or the cell needs to use precursors to produce other metabolites, SREBP is prevented from translocating to the nucleus and binding to SRE, causing the production of HMG-CoA reductase to be attenuated.
HMG-CoA reductase is regulated at additional levels: Glucocorticoids (metabolites of cholesterol) and other compounds accelerate HMGCR mRNA breakdown. In addition, HMG-CoA reductase is regulated by sterol-accelerated ubiquitination and degradation. However, no total blockade of the enzyme occurs, since sufficient intermediates have to be supplied for the synthesis of non-sterols. Similar regulation is exerted with HMG-CoA synthase and mevalonate kinase.
Transport of Cholesterol
Lipoproteins are spherical complexes of lipid and protein, which include chylomicrons, very-low density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL). These all differ in their lipid and protein compositions, size, density, and site of biosynthesis. Lipoproteins function in transport of lipids between tissues. Cholesterol is one of the lipids that are transported, and as a result of their less than perfect transport, there is a gradual deposition of cholesterol in tissues, which is a potentially life-threatening condition when plaques form in blood vessels.
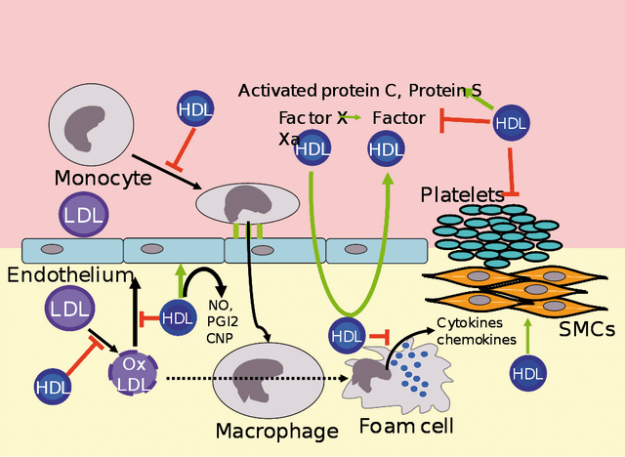
Metabolism of HDL
Lipoproteins are composed of an endogenous or exogenous triacylglycerol or cholesteryl esters forming a core surrounded by shell composed of apolipoproteins, phospholipids, or free cholesterol. The polar portions of the lipids are oriented so that they are on the surface of the lipoprotein, and this makes them soluble.
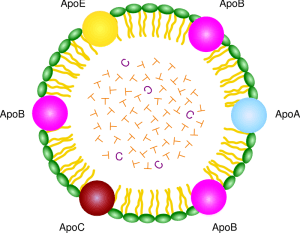
Structure of LDL
Chylomicrons are lipoprotein particles with the lowest density and largest in size; in other words, they contain the highest percentage of lipid and the lowest percentage of protein. VLDLs and LDLs are successively denser, having higher ratios of protein to lipid. HDL particles are the densest.
The apolipoprotein portion associated with lipoprotein particles has a number of diverse functions, such as providing recognition sites for cell-surface receptors, and as activators of lipoprotein metabolism. They are divided by structure and function into five major classes, A through E, with most classes having subclasses, for example, apolipoprotein (or apo) A-I and apo C-II.
Elimination of Cholesterol
After LDL enters the liver, cholesterol esters are hydrolyzed lysosomal cholesterol esterase. Excess cholesterol produced in this way activates acetyl-CoA cholesterol acyl-transferase (ACAT), which acts to re-esterify cholesterol into cholesterol esters that can be deposited as lipid droplets, or mobilized again to where it is needed.
Clinical Correlation
Disturbances in cholesterol metabolism cause various diseases. In familial hypercholesterolemia the uptake of cholesterol into the cells by LDL receptors is diminished. Therefore, in spite of high plasma cholesterol concentration, (intracellulary regulated) cholesterol synthesis still proceeds at high speed.
Comentários
Enviar um comentário