Aging: Mitochondrial Dysfunction and Telomere Attrition
Table of Contents
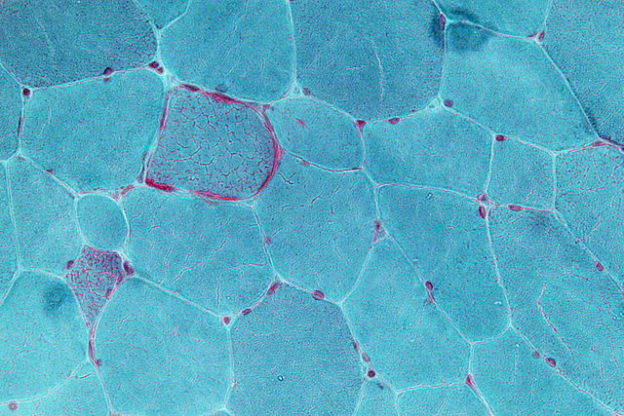
Image: “Very high magnification micrograph showing ragged red fibres (also ragged red fibers), commonly abbreviated RRF, in a mitochondrial myopathy. Gomori trichrome stain.” by Nephron. License: CC BY-SA 3.0
Introduction
Aging is defined as a diminished response to stress, escalation of homeostatic imbalance and an enhanced threat from aging-related pathologies.
There are various diversified theories propagated to explain aging. They can be enlisted as follows:
- Programmed theory
- Stochastic theory
- Molecular gene theory
- Evolutionary theory
- Cellular theory
- System theory
These theories reflect the unparalleled boom in scientific research and knowledge and a refinement in our understanding of the biomedical pathogenesis of aging. Various characteristics of aging can be summarized as follows:
- Trait
- Genomic instability
- Mitochondrial dysfunction
- Telomere attrition
- Epigenetic alterations
- Loss of proteostasis
- Altered intercellular communication
- Cellular senescence
- Deregulated nutrient sensing
- Stem cell exhaustion
We now discuss mitochondrial dysfunction and telomere attrition in detail.
Mitochondrial Dysfunction
The word “mitochondria” has its humble origins in Greek literature, where mitos stands for thread and chondrion reflects the granular appearance. Also known as the “powerhouse” of the cell, the mitochondria is ubiquitously present in almost all eukaryotic cells. Few exceptions are well known, such as the RBCs (red blood cells). Mitochondria generate ATP – the chemical energy currency of every cell.
Aberrations of mitochondrial functions are implicated in many disorders, such as heart diseases, autism, and mitochondrial disorders, as also in aging. Being the center stage for cellular energy pathways, mitochondria are also subjected to production and subsequent molecular damage by free radicals, produced as a byproduct of the vast energy turnover reactions that occur in every cell. Proton drive across ATP synthase leads to energy production. Naïve proton leak through these complex gates of the respiratory chain leads to the production of superoxide radicals.
Image: “In chemistry, a free radical is an atom, molecule, or ion with an unpaired valence electron.” by Healthvalue. License: CC BY-SA 3.0
Regulated by positive feedback, free radical injury leads to mitochondrial damage and increase in oxidative stress, which, in turn, escalates production of the free radicals, ultimately leading to the consummation of the entire cell.
These ideas are reflected in an organized manner in the mitochondrial dysfunction theory of cellular aging. One of the major theories of aging, which has stood the test of time, is the Free Radical Theory.
One very peculiar characteristic of mitochondria is the presence of its own customized genome which brings about the transcription of mitochondrial proteins.
A strict, highly regulated, but dynamic control is maintained on the mitochondrial genome. It is not without its limitations and is susceptible to error about 15 times as compared to the nuclear genome. Somatic mutations in the mitochondrial genome have been extensively studied and are thought to be incriminated in neurodegenerative diseases. Claims of the contribution of somatic mutations in aging with subsequent diminution of mitochondrial homeostasis abound.
Damage to the mitochondrial genome is implicated due to the following observations:
- The absence of histone protective layer around mitochondrial DNA
- Limited repair mechanisms in mitochondrial DNA
- Location of the mitochondrial genome in the inner mitochondrial membrane which is near the respiratory chain and, thus, paradoxically near to the location of origin of free radicals which can easily harm the genome.
However, these claims are not accepted unequivocally. On the other hand, there are studies which reveal that mitochondrial DNA mutation bulk might be quite stationary and stagnant in an individual’s lifetime and might, in fact, not be affected by the production of reactive oxygen species and free radical molecules.
There is also the presence of seemingly contradictory evidence to suggest perhaps, the radical oxygen species might, in fact, prolong the lifespan. They are postulated to bring about the same by triggering survival in response to physiological stress. Free radicals may increase in parallel with mitochondrial attempts to maintain survival.
Regeneration mistakes in mitochondrial DNA
Another fascinating, upcoming recent theory expatriates on the importance of spontaneously occurring regeneration mistakes in mitochondrial DNA which culminate in mutations that dictate a decline in a cell’s vitality and function.
Recent evidence has emerged that suggests the mitochondria undergo independent replication from the cell replication process. This means that a single mitochondrion might undergo multiple replication cycles while another one might not undergo replication at all! This information is very important for the experimental biologist because it can give us new insights into the role of mitochondria in health and disease.
Recently, it was suggested that mitochondrial DNA mutations might occur during embryogenesis. When these mutations are not inherited, they affect a small number of the total mitochondrial population of our cells. Clonal expansion of these mutated mitochondria occurs, and such mutated mitochondria tend to segregate within the same cell.
Eventually, a significant number of mutated mitochondria are segregated in a large number of cells. Cells with a significant number of mutated mitochondria demonstrate impaired respiratory chain dysfunction and eventually undergo apoptosis.
Whether acquired or inherited, mitochondrial DNA replicative errors have the same effect on the cell function which can be summarized as follows:
Mitochondrial dysfunction is also considered an important aspect of other biomedical theories about aging. The same can be tabulated as follows:
The Limitations of our Understanding of Mitochondrial Dysfunction in Aging
There are many problems in our current understanding of:
- How mitochondrial dysfunction occurs in the aging cell
- How this dysfunction can affect the cell
Moreover, our current approaches in assessing mitochondrial function and dysfunction are limited. The following points summarize the current limitations related to mtDNA mutations in mitochondrial dysfunction in aging:
While mutations in mitochondrial DNA appear to play a role in aging, recent evidence has emerged that shows the mitochondrial morphology, shape, and motility are also impaired in aging even without any DNA changes. In fact, some mitochondrial diseases have been proven to be related to the shape and configuration of the mitochondria more than the mutation itself.
Recent evidence has emerged that inherent errors in the Pol Y gene are responsible for almost all the mutations that occur in mitochondrial DNA replication due to aging. This finding completely contradicts the hypothesis that spontaneous replication errors create most of the mitochondrial DNA mutations.
The exact role of the respiratory chain dysfunction in health and disease is still poorly understood.
Telomere Attrition
Telomere shortening and its association with aging have invigorated a new life in the management of aging as lifestyle modifications are thought to affect telomere length and, thus subsequently, aging. Progressive truncation of telomere length has several repercussions.
The same has been tabulated as follows:
- Aggravation in a frequency of age-related diseases
- Senescence
- Malignant transformation of stem cells
- Apoptosis
- Poor survival
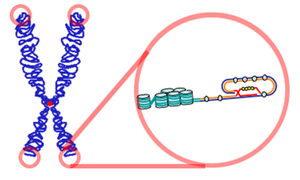
Image: “Telomeres are found at the termini of chromosomes. The end of a telomere inserts back into the main body of the telomere to form a T-loop.” License: CC BY-SA 3.0
Telomeres are complex associates with proteins and DNA which are stationed at chromosomal ends mainly to subserve chromosomal protection. Telomeres shield chromosomes from a multitude of exogenous and endogenous threats through mechanisms listed as follows:
- Inhibition of exonucleolytic degradation through TRF2
- Priming of telomeric DNA synthesis through mechanisms homologous to gap filling mechanisms
- Prevention of extraneous chromosomal recombination
- Avoidance of unnecessary DNA repair
- Deterrence of interchromosomal fusion
- Recombinational repair
Telomerase bestows upon cells’ ability to maintain telomere length and hence replicate without being pushed towards a decline in vitality and function. Somatic cells have a minuscule level of telomerase activity. Substantial telomerase function has been documented in germline cells and stem cells.
In human beings, an average cell loses telomere length at a rate of about 24 to 27 base pairs per year. When the telomere length decreases below a certain critical threshold, the cell undergoes aging. The Hayflick limit is the number of times a human cell can divide. Most mammalian somatic cells do not express telomerase. 50 divisions are the limit.
Thus, telomere serves as a biological clock meant to ordain the lifespan of an individual.
Various lifestyle factors influence attrition of telomere length. They can be summarized as follows:
- Smoking
- Body weight
- Exercise
- Age
- Stress
- Social and economic status
Gender does not affect telomere shortening in a significant manner.
Dyskeratosis congenita is a genetic disease characterized by progeroid features, such as untimely graying of hair, susceptibility to infections and insidious bone marrow failure. It is marked by an escalated vulnerability to develop cancers. The root cause of this dreadful illness can be traced to a deficiency of the telomerase RNA gene.
Telomere attrition is a cause of cellular damage, also known as a primary hallmark of aging. Mitochondrial dysfunction is a response to damage, also known as the antagonistic hallmark of aging. Hallmarks of aging can be summarized as follows:
Summary
Aging is defined as a diminished response to stress, escalation of homeostatic imbalance and the enhanced threat from aging-related pathologies.
There is a multitude of theories and speculations proposed and various traits of aging identified to better expound on this mysterious but inevitable phenomenon. None of them, however, enjoy unequivocal evidence to explain aging.
Mitochondrial dysfunction and Telomere attrition are important contributors to the decline in a cell’s vigor, vitality and functioning, ultimately leading to senescence.
Mitochondria are termed as the powerhouse of a cell. Along with the generation of the energy currency of a cell, ATP, mitochondria inadvertently also give rise to free radicals. Free radicals can potentially cause nuclear and mitochondrial genomic instability, severe damage to the cellular homeostasis and aging.
Mitochondria possess their own customized mitochondrial DNA. It is prone to damage in a multitude of ways and can lead to aging.
Mitochondrial dysfunction is an integral component of replicative senescence theory concerning the stem cells. It is also implicated in sarcopenia.
Mutator mice is a model created to determine the potential causal relationship between mitochondrial dysfunction, genomic instability and aging.
Telomeres are complex molecules aggregated at the end of chromosomes to protect the latter from inadvertent recombination, genomic instability, and subsequent cellular damage, especially during replication.
Telomeres progressively shorter as the cell divides and this attrition reaches a threshold after which further division has more risks and lesser benefits; thus, they serve as biological clocks. Telomere attrition is implicated in aging, increased predisposition to cancer and age-related diseases.
Lifestyle modifications affect the telomere length and hence the emphasis on lifestyle modification to stall aging.
Review Questions
The correct answers can be found below the references.
1. Mitochondrial dysfunction is implicated in which of the following conditions?
- Sarcoma
- Leucopenia
- Sarcopenia
- Chug Strauss syndrome.
2. Which of the following statements about “mutator mice” is true?
- Also known as Polg M mice, these have been developed to possess heterozygous knock-in mutation for mitochondrial DNA polymerase enzyme.
- Also known as Polg A mice, these have been developed to possess homozygous knock-in mutation for mitochondrial DNA polymerase enzyme.
- Also known as Polg A mice, these have been developed to possess homozygous knock-in mutation for mitochondrial DNA synthetase enzyme.
- Also known as Polg M mice, these have been developed to possess homozygous knock-in mutation for mitochondrial DNA synthetase enzyme.
3. Which of the following genetic disorders is secondary to defect in the normal functioning of telomerase enzyme?
- Weber syndrome
- Von Recklinghausen syndrome
- Dyskeratosis corpora
- Dyskeratosis congenital

Comentários
Enviar um comentário