Water and Sodium Balance and Their Associated Disorders
Table of Contents
- Fluid Compartments of the Body
- Difference between the Composition of ECF and ICF
- Osmosis
- Osmolality and Osmolarity
- Plasma Osmolality
- Clinical Significance of Plasma Osmolality
- Regulation of Extracellular Fluid Composition & Volume
- Disorders Causing Hypernatremia
- Treatment of Hypernatremia
- Disorders causing Hyponatremia
- Treatment of Hyponatremia
- Review Questions
- References
Fluid Compartments of the Body
There is normally a continuous exchange of body fluids and solutes with the external environment and different body compartments of the body. The properties of extracellular fluid (ECF) and intracellular fluid (ICF) are summarized in the following table:
Difference between the Composition of ECF and ICF
Extracellular fluid (ECF)
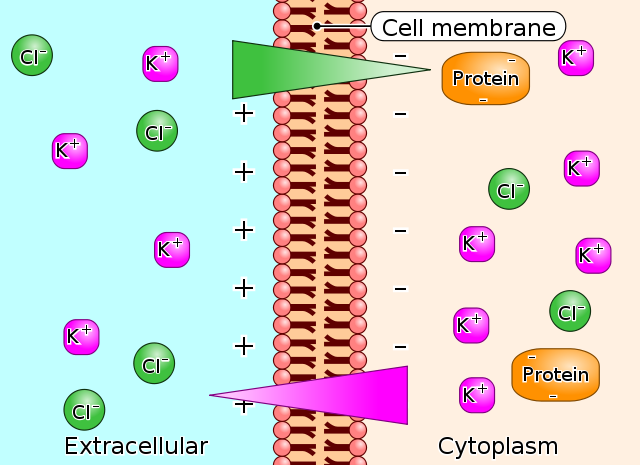
There is a highly permeable membrane between the plasma and interstitial fluid, allowing the continuous exchange of solutes between the two compartments. The membrane has a low permeability to proteins, allowing only a small amount of proteins to be leaked from the plasma to the interstitial fluid. This makes the concentration of proteins in plasma higher than interstitial fluid.
Donnan effect
The large anionic proteins in plasma make a net negative charge, therefore attracting the positively charged ions (cations), such as potassium and sodium ions; thus, holding an extra amount of cations in the plasma along with protein.
Image: “Gibbs-Donnan-Effect” by Biezl. License: Public Domain
The negative charges of protein in plasma repel the negatively charged ions (anions), such as chloride ions; thus, there is a slightly higher concentration of anions in interstitial fluid than the plasma. Because of the Donnan effect, the concentration of cations is about 2% greater in the plasma than interstitial fluid.
Extracellular fluid contains a high concentration of:
- Sodium ions
- Chloride ions
- Bicarbonate ions
Extracellular fluid contains a low concentration of:
- Potassium
- Calcium
- Magnesium
- Phosphate
- Organic acid ions
The compositions of extracellular fluid are regulated chiefly by the kidneys (discussed in a later section). The ECF balance between plasma and interstitial fluid is determined chiefly by the balance of hydrostatic and colloid osmotic forces across the capillary membranes.
Intracellular fluid (ICF)
There is a highly permeable cell membrane which separates the ECF from the ICF, allowing the water to diffuse easily from interstitial fluid into the cells. In contrast to ECF, intracellular fluid contains a high concentration of:
- Potassium ions
- Phosphate ions
- Magnesium ions (moderate amount)
- Sulfate ions (moderate amount)
Intracellular fluid contains a low concentration of:
- Sodium ions
- Chloride ions
- Calcium ions
Also, the ICF contains a large amount of protein, which is four times the amount of plasma protein.
Distribution of fluid between ICF and ECF is determined mainly by the osmotic effect ofsodium and chloride, which are the most osmotically active solutes acting across the cell membrane. The concentration of sodium in ECF is the most important determinant of ECF volume because changes in concentration of chloride are, to a greater extent, secondary to changes in the concentration of sodium.
The water moves rapidly across the cell membrane by this osmotic effect to keep the ECF isotonic with the ICF.
Osmosis
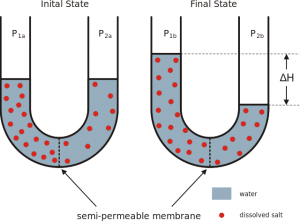
Osmosis is the net diffusion of water across a selectively permeable membrane from a region of low solute concentration (high water concentration) to one with a high solute concentration(low water concentration).
Image: “An example of osmosis: dissolved salt forcing water to pass through a semi-permeable membrane” by Hans Hillewaert. License: CC BY-SA 3.0
Osmolality and Osmolarity
The total number of particles in a solution is measured in Osmoles.
One osmole (osm) = 1 mole (mol) of solute particles
For example, a solution containing 1 mole of glucose in each liter has a concentration of 1 osm/L.
Osmolality is the osmolal concentration of a solution when it is expressed as osmoles/kg of water. Osmolarity is the osmolal concentration of a solution when it is expressed as osmoles/L of water. About 80% of total osmolarity of the interstitial fluid and plasma is due to sodium and chloride ions, while half of the total osmolarity of the intracellular fluid is due to potassium ions and the remainder is due to other intracellular substances.
Plasma Osmolality
POsm = 2 (serum Na+ ) + (serum glucose/18) + (serum blood urea nitrogen (BUN)/(2.8)
= 280 – 295 mOsm/kg
There is an osmotic equilibrium between intracellular and extracellular fluids, and even small changes in the concentration of these impermeant solutes in extracellular fluids can make a large osmotic pressure across the cell membrane, with subsequent rapid movement of water across the cell membrane.
- If the plasma osmolality is high (hypertonic state), water will diffuse out of the cells to the ECF, and will result in cell shrinkage.
- If the plasma osmolality is low (hypotonic state), water will diffuse into the cells, and will result in cell swelling.
- If the osmolality is equal between ICF & ECF (isotonic state), water will not diffuse across the cell membrane and the cells will not shrink nor swell.
Macromolecules (mainly proteins) determine the so called oncotic pressure. Intravasal situated Albumin keeps water in plasma. In the case of a loss of Albumin (cirrhosis, nephrotic syndrome), the oncotic pressure of the plasma decreases and water moves into the interstitium. Edemas will be the outcome.
The hydrostatic pressure is mainly determined by the blood pressure. Decreased venous return (e.g. right heart failure) increases the hydrostatic pressure and water gets pressed out of the vessels into the interstitium which, as well, then leads to edemas.
Clinical Significance of Plasma Osmolality
Decreased plasma sodium concentration
Decreased plasma sodium concentration (Hyponatremia < 135 mEq/L) occurs from either:
- Loss of NaCl from ECF or
- Additional excess water to the ECF
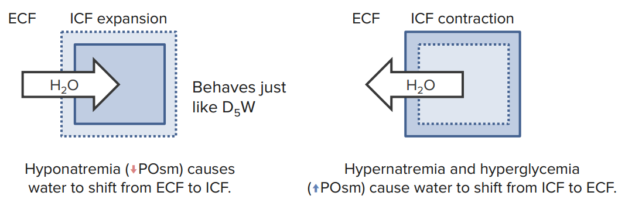
Loss of NaCl from ECF results in hypo-osmotic (hyponatremic/hypotonic) dehydration, in which the water moves from the region of low solute concentration (plasma) to the region of high solute concentration (intracellular), leading to:
1. Decrease in ECF, with signs and symptoms of volume depletion:
- Orthostatic hypotension (defined as a decrease in systolic blood pressure of 20 mm Hg or a decrease in diastolic blood pressure of 10 mm Hg within three minutes of standing when compared with blood pressure from the sitting or supine position), is the earliest sign of volume depletion
- Reflex tachycardia with weak pulse
- Delayed capillary refilling
- Dry mucus membranes (as dry tongue)
- Cold extremities
- Oliguria
2. Expansion of ICF, which results in cell swelling and edema. The most dangerous is brain edema, which may lead to lethargy and coma. Furthermore:
- Rapid correction of hypernatremia can create a new gradient that causes water movement from the extracellular space into the cells of the brain; this can produce cerebral edema.
- Avoid more than a 12-mEq/L decrease in the serum Na every 24 hours.
Increased plasma sodium concentration
Increased plasma sodium concentration (Hypernatremia > 145 mEq/L) occurs from either:
- Loss of water from the ECF or
- Additional excess sodium to the ECF
Loss of water from ECF results in Hyper-osmotic (hypernatremic/hypertonic) dehydration, in which the water moves from the region of low solute concentration (intracellular) to the region of high solute concentration (plasma) resulting in:
1. Increase in ECF, making the clinical signs of dehydration (e.g. signs of shock) less severe.
2. Decrease in ICF, which leads to cell shrinkage:
- Making the mucus membranes severely dry (woody tongue).
- Shrinkage of brain cells may result in seizures and coma.
- Rapid correction of hyponatremia can produce central pontine myelinolysis.
- Avoid more than a 12-mEq/L/24 increase in the serum Na every day, especially if the hyponatremia developed gradually.
When proportionally the same amount of water and sodium are lost from the body, the sodium concentration in the ECF will not change, resulting in iso-osmotic (normonatremic/isotonic) dehydration. Therefore, the mechanisms that control Na+ balance are the major mechanisms that control ECF volume.
Summary
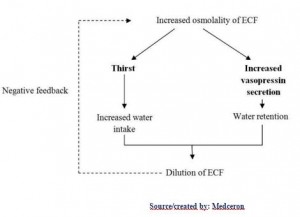
Regulation of Extracellular Fluid Composition & Volume
The two main mechanisms in the regulation of the ECF osmolality, and sodium concentration are:
- Antidiuretic hormone (vasopressin) secretion
- Thirst mechanism
Anti-diuretic hormone (vasopressin) secretion
Antidiuretic hormone (ADH), also called vasopressin, is a very important mechanism that regulates the plasma osmolality and sodium concentration by altering the water excretion by the kidneys.
It is synthesized in the hypothalamus and stored in the posterior pituitary. They are released into the bloodstream in response to various stimuli. Its secretion is regulated by osmoreceptors which are located in the anterior hypothalamus.
The stimuli of the ADH secretion are:
1. Osmotic stimulus
When the osmolality (plasma Na concentration) rises above the normal 285 mOsm/kg, as in the case of water deficit, this causes shrinkage of osmoreceptors in which signals are transmitted to the posterior pituitary and stimulates release of ADH into the bloodstream to reach the kidney, and vice versa.
2. Volume stimulus
When the volume of ECF is decreased, this stimulates stretch receptors in the low- and high-pressure portions of the vascular system, in which impulses pass to the posterior pituitary and stimulates the release of ADH. Low-pressure receptors are present in the great veins, right and left atria, and pulmonary vessels. High-pressure receptors present in the carotid sinuses and aortic arch.
3. Blood Pressure/volume stimulus
The decrease of arterial pressure and/or blood volume, as in the case of the hypovolemia, stimulates the release of ADH, as well as activation of the Renin-Angiotensin system, whichstimulates aldosterone secretion and promotes sodium and water retention (discussed later in details).
ADH reaches the kidney and increases the permeability of the late distal tubules and collecting ducts, so the water diffuses into the hypertonic interstitium of the renal pyramids. This results in the reabsorption of water into the bloodstream, while the sodium and other solutes are continuously excreted in the urine. This causes dilution of solutes in ECF and correction of the high osmolality, and forming concentrated urine.
The opposite of these sequences occurs when there is low osmolality of ECF. (Less ADH is secreted which leads to a decrease of water permeability of late distal tubules and collecting ducts, resulting in an increase of water excretion (this results in an increase of the ECF osmolality), and formation of a diluted urine.)
Thirst mechanism
Increased ECF osmolality causes a shrinkage of the thirst center (located anterolaterally in the preoptic nucleus), resulting in stimulating thirst sensation (thus increases fluid intake and dilutes high ECF osmolality to the normal, and vice versa).
Both osmoreceptor-ADH secretion and thirst mechanisms work simultaneously to keep the ECF osmolality and volume within normal levels. Here you have a summary of different factors that affect the regulatory mechanisms of ECF composition and volume:
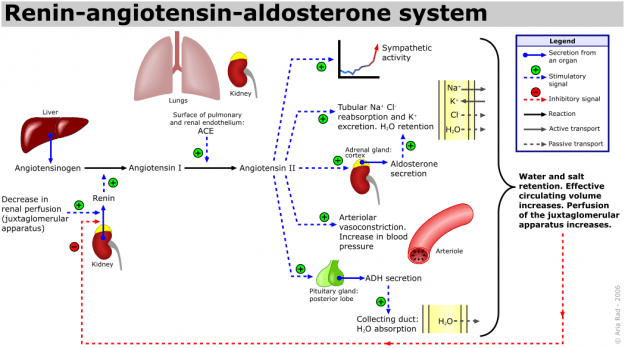
Role of Renin-Angiotensin-Aldosterone System
Image: “The renin-angiotensin system (RAS) or the renin-angiotensin-aldosterone system (RAAS). Start reading this schematic from the left, where it says “Decrease in renal perfusion (juxtaglomerular apparatus)”. Alternatively, the RAAS can also be activated by a low NaCl concentration in the macula densa or by sympathetic activation.” by A. Rad. License: CC BY-SA 3.0. The blue and red dashed arrows indicate stimulatory or inhibitory signals, which is also indicated by the +/-. In the tubule and collecting duct graphics, the grey dashed arrows indicate passive transport processes, contrary to the active transport processes which are indicated by the solid grey arrows. The other solid arrows either indicate a secretion from an organ (blue, with a starting spot) or a reaction (black). These 2 processes can be stimulated or inhibited by other factors.
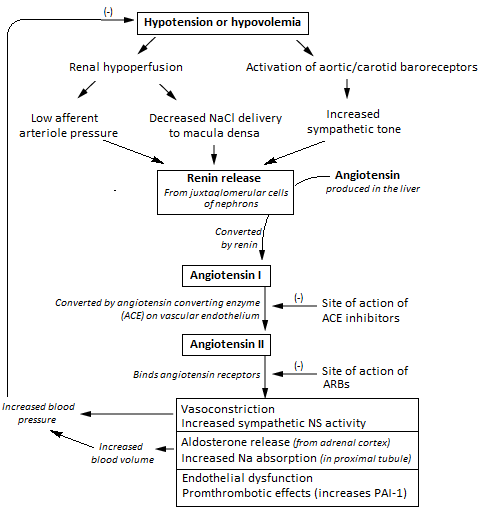
Angiotensin II and aldosterone regulate the concentration of sodium in extreme conditions by altering the reabsorption of sodium by the renal tubules. This results in altering the water reabsorption, along with the sodium. Thus, activation of this system leads to an increase of both the sodium quantity and ECF volume (hence also a little change in the sodium concentration because the water is absorbed along with sodium, a process called as sodium and water retention.
When the blood pressure/volumes falls, the juxta-glomerular apparatus of the kidneys start to release renin, which passes out of the kidney and enters into the circulation. The renin then acts enzymatically on the angiotensinogen to release angiotensin I.
Within a few seconds to minutes after the formation of angiotensin I, it is converted to angiotensin II by the effect of angiotensin converting enzyme (ACE) that is present in the endothelium of the lung vessels.
Angiotensin II has two principal effects that can elevate arterial pressure:
- Firstly, it causes vasoconstriction of the arterioles in many areas in the body (resulting in an increase in the total peripheral resistance, thereby raising the arterial pressure).
- Secondly, it decreases the excretion of both salt and water by the kidneys through stimulating the adrenal glands to secrete aldosterone. Aldosterone increases the absorption of sodium and water by the renal tubular cells (causing Na and water retention and simultaneously increases excretion of potassium and H ions).
Image: “Flowchart of the renin angiotensin aldosterone system and its clinical effects.” by Npatchett. License: CC BY-SA 4.0
Disorders Causing Hypernatremia
(Serum Na < 146 mEq/L): always a hyperosmolar state
1. Net water loss
Hypotonic fluid
Renal losses:
- Loop diuretics
- Osmotic dieresis
- Postobstructive dieresis
- Intrinsic renal disease
Non-renal losses:
- Vomiting
- NG suctioning
- Enterocutaneous fistula
- Burns
- Excessive sweating
Diabetes Insipidus
- Central DI (low ADH secretion, causes: idiopathic 50%/head trauma/destructive diseases)
- Nephrogenic DI ( unresponsiveness of renal tubules to ADH, acquired: chronic lithium use (most)/hypercalcemia/UTI (pyelonephritis), can be congenital)
Diagnosis:
- Urine: ↓ Specific Gravity, ↓ Osmolality
- Plasma osmolality: 280 to 310 mOsm/kg
- Water deprivation test (withhold fluids and measure urine osmolality every hour; When urine osmolality is stable (< 30 mOsm/kg hourly increase for 3 hours), inject 2 g desmopressin S.C. and measure urine osmolality 1 hour later)
2. Hypertonic Sodium Gain
Primary hyperaldosteronism
Increase aldosterone secretion from the adrenal gland.
Causes:
- Functioning adenoma: “Conn Syndrome”
- Bilaternal Adrenal Hyperplasia
Clinical picture:
- ↑ Na & Water → Hypertension
- ↑ K & H ions → Hypokalemia & M. Alkalosis
- Polydipsia, nocturnal polyuria (due to hypokalemia)
- Absence of peripheral edema
Diagnosis:
- Screening: Plasma: ↑ aldosterone/↓ Renin/A:R ratio>30
- Confirmation: with either 1) Saline infusion test (Infusion of saline will decrease aldosterone levels in normal patients) or 2) Oral Sodium Loading
Cushing syndrome
Cushing syndrome means an abnormal increase in cortisol level.
Causes:
- A) Iatrogenic: exogenous steroids (most common)
- B) Non-iatrogenic:
- ACTH independent → from adrenal gland (primary)
- ACTH dependent→ secondary: ↑ ACTH From pituitary (“Cushing’s disease”) and ectopic ACTH production (as in small cell carcinoma (SSC) of lung)
Clinical picture
- Changes in appearance: central obesity, hirsutism, moon facies, “buffalo hump,” purple striae on abdomen, acne
- Hypogonadism, masculinization in females, proximal muscle wasting and weakness, psychiatric disturbances
Diagnosis:
- Screening:
- 1) low-dose dexamethasone suppression test: if the serum cortisol is < 5 → No Cushing; if the serum cortisol is > 5 → Cushing syndrome
- 2) 24-hour urinary free cortisol level
- ACTH level:
- If low→ 1ry Cushing (adenoma or hyperplasia)
- If high→ 2ry (pituitary or ectopic production)
- To differentiate between pituitary & ectopic ACTH: High dose dexa suppression test (in Pituitary → decrease in cortisol > 50% | in ectopic → No suppression occur)
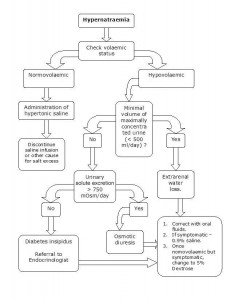
Treatment of Hypernatremia
Image: “Management of Hypernatremia” by HarishV. License: CC BY-SA 3.0
If there is circulatory compromise, this should be treated with isotonic saline to correct the water deficit.
In patients without circulatory compromise, they should be treated with hypotonic solutions.
If hypernatremia developed in hours, rapid correction at a rate of 1 mEq/L/h can be done with no risk of brain edema. However, if the time of development of hypernatremia is unknown, the rate of correction should be 0.5 mEq/L/h and the correction should not exceed 10–12 mEq/L in 24 hours to avoid brain edema.
Disorders causing Hyponatremia
(Serum Na < 136 mEq/L): it can be hyper-,hypo-, isotonic
Isotonic hyponatremia
Hyperlipidemia, hyperproteinemia and hyperglycemia can cause a laboratory artifact as a result of a decreased water component of the plasma, so it is needed to rule out the spurious hyponatremia at first.
Hypertonic hyponatremia
It occurs in hyperosmolar states, in which a large amount of impermeable solutes can’t cross cell membranes, such as mannitol and glucose (hyperglycemia). In prolonged uncorrected hyperglycemia, the hyperosmolality can withdraw water into ECF, diluting the Na and results in hyponatremia. Hypernatremia can also occur if osmotic diuresis developed.
Hypotonic hyponatremia
It is classified according to the volume status of the body into:
- Euvolemic hyponatremia
- Hypovolemic hyponatremia
- Hypervolemic hyponatremia
Euvolemic hyponatremia
- If urine osmolality < 100 mOsm/kg: indicates “Psychogenic Polydepsia”
- If urine osmolality > 100 mOsm/kg: indicates that urine is inappropriately concentrated, occurs in the following:
Hypovolemic Hyponatremia
- Urine Na < 20 mEq/L
- Vomiting
- Diarrhea
- Nasogastric tube
- Sequestration of fluid in: burns, ileus, traumatized muscle, pancreatitis
- Urine Na > 20 mEq/L:
Hypervolemic Hyponatremia
Treatment of Hyponatremia
Urgent treatment
It’s indicated in:
- Severe hyponatremia (Sodium < 120 mEq/L and symptoms: mental status changes, seizure, or coma)
- Abrupt hyponatremia developed
- Should be treated with infusion of 3% hypertonic saline solution, and should be corrected at rate of 1 mEq/L/h for the first 3–4 hours or until symptoms resolve, with a rate of correction less than 10–12 mEq/L in 24 hours to avoid Osmotic demyelination in case of rapid correction
Emergency dialysis is indicated in patients with:
- Renal failure
- Volume overload
- Severe hyponatremia
Non-urgent treatment
Those who don’t need urgent treatment can be treated with fluid restriction with an isotonic saline replacement to avoid circulatory compromise, together with continuous observation of the patient. Do not forget to treat the underlying cause, e.g. hormonal replacement in adrenal insufficiency.
Review Questions
The answers are below the references.
1. A 78-year-old male is admitted to the hospital. He is presented with severe hyponatremia (sodium < 120 mEq/L). Other symptoms observed in the patient are nausea, malaise, lethargy, headache, seizures and coma. The most appropriate treatment for the patient is…
- …infusion of 3% hypertonic saline solution.
- …infusion of 6% hypertonic saline solution.
- …infusion of 3% mannitol solution.
- …infusion of 3% glucose solution.
- …infusion of 3% hypotonic solutions.
2. What is the correct definition of osmosis?
- Osmosis is the net diffusion of water across a selectively permeable membrane from a region of low solute concentration to one with a high solute concentration.
- Osmosis is the net diffusion of water across a selectively permeable membrane from a region of high solute concentration to one with a low solute concentration.
- Osmosis is the osmolal concentration of a solution when it is expressed as osmoles/Kg of water.
- Osmosis is the osmolal concentration of a solution when it is expressed as osmoles/L of water.
- Osmosis is the property of a system in which variables are regulated so that internal conditions remain stable and relatively constant.
3. The osmotic pressure and compositions of extracellular fluid are regulated chiefly by which of the following organ of the body?
- Liver
- Heart
- Lungs
- Villi of the intestine
- Kidney

Comentários
Enviar um comentário